A Novel N-Tetrasaccharide in Patients with Congenital Disorders of Glycosylation, Including Asparagine-Linked Glycosylation Protein 1, Phosphomannomutase 2, and Mannose Phosphate Isomerase Deficiencies
- PMID: 26430078
- PMCID: PMC4819965
- DOI: 10.1373/clinchem.2015.243279
A Novel N-Tetrasaccharide in Patients with Congenital Disorders of Glycosylation, Including Asparagine-Linked Glycosylation Protein 1, Phosphomannomutase 2, and Mannose Phosphate Isomerase Deficiencies
Abstract
Background: Primary deficiencies in mannosylation of N-glycans are seen in a majority of patients with congenital disorders of glycosylation (CDG). We report the discovery of a series of novel N-glycans in sera, plasma, and cultured skin fibroblasts from patients with CDG having deficient mannosylation.
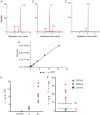
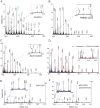
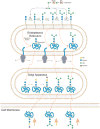
Method: We used LC-MS/MS and MALDI-TOF-MS analysis to identify and quantify a novel N-linked tetrasaccharide linked to the protein core, an N-tetrasaccharide (Neu5Acα2,6Galβ1,4-GlcNAcβ1,4GlcNAc) in plasma, serum glycoproteins, and a fibroblast lysate from patients with CDG caused by ALG1 [ALG1 (asparagine-linked glycosylation protein 1), chitobiosyldiphosphodolichol β-mannosyltransferase], PMM2 (phosphomannomutase 2), and MPI (mannose phosphate isomerase).
Results: Glycoproteins in sera, plasma, or cell lysate from ALG1-CDG, PMM2-CDG, and MPI-CDG patients had substantially more N-tetrasaccharide than unaffected controls. We observed a >80% decline in relative concentrations of the N-tetrasaccharide in MPI-CDG plasma after mannose therapy in 1 patient and in ALG1-CDG fibroblasts in vitro supplemented with mannose.
Conclusions: This novel N-tetrasaccharide could serve as a diagnostic marker of ALG1-, PMM2-, or MPI-CDG for screening of these 3 common CDG subtypes that comprise >70% of CDG type I patients. Its quantification by LC-MS/MS may be useful for monitoring therapeutic efficacy of mannose. The discovery of these small N-glycans also indicates the presence of an alternative pathway in N-glycosylation not recognized previously, but its biological significance remains to be studied.
© 2015 American Association for Clinical Chemistry.
Conflict of interest statement
Figures

Comment in
-
Protein-Specific Glycoprofiling for Patient Diagnostics.Clin Chem. 2016 Jan;62(1):9-11. doi: 10.1373/clinchem.2015.248518. Epub 2015 Nov 19. Clin Chem. 2016. PMID: 26585926 No abstract available.
References
-
- Lacey JM, Bergen HR, Magera MJ, Naylor S, O'Brien JF. Rapid determination of transferrin isoforms by immunoaffinity liquid chromatography and electrospray mass spectrometry. Clin Chem. 2001;47:513–8. - PubMed
-
- Xia B, Zhang W, Li X, Jiang R, Harper T, Liu R, Cummings RD, He M. Serum N-glycan and O-glycan analysis by mass spectrometry for diagnosis of congenital disorder of glycosylation. Anal Biochem. 2013;442:178–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

