Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib
- PMID: 26430171
- PMCID: PMC4666333
- DOI: 10.3324/haematol.2015.126672
Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib
Abstract
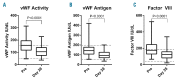
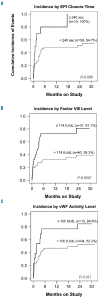
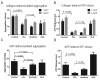
Ibrutinib is associated with bleeding-related adverse events of grade ≤ 2 in severity, and infrequently with grade ≥ 3 events. To investigate the mechanisms of bleeding and identify patients at risk, we prospectively assessed platelet function and coagulation factors in our investigator-initiated trial of single-agent ibrutinib for chronic lymphocytic leukemia. At a median follow-up of 24 months we recorded grade ≤ 2 bleeding-related adverse events in 55% of 85 patients. No grade ≥ 3 events occurred. Median time to event was 49 days. The cumulative incidence of an event plateaued by 6 months, suggesting that the risk of bleeding decreases with continued therapy. At baseline, von Willebrand factor and factor VIII levels were often high and normalized on treatment. Platelet function measured via the platelet function analyzer (PFA-100™) was impaired in 22 patients at baseline and in an additional 19 patients on ibrutinib (often transiently). Collagen and adenosine diphosphate induced platelet aggregation was tested using whole blood aggregometry. Compared to normal controls, response to both agonists was decreased in all patients with chronic lymphocytic leukemia, whether on ibrutinib or not. Compared to untreated chronic lymphocytic leukemia patients, response to collagen showed a mild further decrement on ibrutinib, while response to adenosine diphosphate improved. All parameters associated with a significantly increased risk of bleeding-related events were present at baseline, including prolonged epinephrine closure time (HR 2.74, P=0.012), lower levels of von Willebrand factor activity (HR 2.73, P=0.009) and factor VIII (HR 3.73, P=0.0004). In conclusion, both disease and treatment-related factors influence the risk of bleeding. Patients at greater risk for bleeding of grade ≤ 2 can be identified by clinical laboratory tests and counseled to avoid aspirin, non-steroidal anti-inflammatory drugs and fish oils. ClinicalTrials.gov identifier NCT01500733.
Copyright© Ferrata Storti Foundation.
Figures

Comment in
-
Comment on Lipsky et al.: Incidence and Risk Factors of Bleeding-Related Adverse Events in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib.Haematologica. 2016 Mar;101(3):e123. doi: 10.3324/haematol.2015.139030. Haematologica. 2016. PMID: 26928252 Free PMC article. No abstract available.
-
Response to Comment on Incidence and Risk Factors of Bleeding-Related Adverse Events in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib.Haematologica. 2016 Mar;101(3):e124-5. doi: 10.3324/haematol.2015.140558. Haematologica. 2016. PMID: 26928253 Free PMC article. No abstract available.
References
-
- Wiestner A. Targeting B-Cell receptor signaling for anticancer therapy: the Bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31(1):128–130. - PubMed
-
- Buggy JJ, Elias L. Bruton Tyrosine Kinase (BTK) and Its Role in B-cell Malignancy. Int Rev Immunol. 2012;31(2):119–132. - PubMed
-
- Byrd JC, O’Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(13):1278–1279. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

