Performance of Momguard, a new non-invasive prenatal testing protocol developed in Korea
- PMID: 26430657
- PMCID: PMC4588837
- DOI: 10.5468/ogs.2015.58.5.340
Performance of Momguard, a new non-invasive prenatal testing protocol developed in Korea
Abstract
Objective: To evaluate the performance of Momguard, non-invasive prenatal test (NIPT) for detecting trisomy (T) 21, T18, T13, and sex-chromosome abnormalities recently developed in Korea.
Methods: This preliminary study formed part of a large prospective cohort study conducted at Asan Medical Center, Seoul, Korea. Only pregnant women who underwent both NIPT and confirmatory karyotyping were included in this study. NIPT results were compared with those of karyotype analyses.
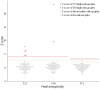
Results: Among 93 eligible cases, NIPT results could not be obtained in one case due to a low fetal cell-free DNA fraction. Based on NIPT, eight cases of fetal aneuploidies, including T21 (n=5), T18 (n=2), and T13 (n=1), were identified. For T21 and T18, the sensitivity and specificity of NIPT were both 100%, with a false-positive and false-negative rate of 0% and a positive-predictive value of 100%. One patient classified as having intermediate risk for T13 by NIPT was confirmed to have T13 by karyotyping, and there were no false-negative cases. No cases of sex-chromosome anomalies were detected by NIPT or karyotyping during the study period.
Conclusion: Momguard is a reliable screening tool for detecting T21 and T18. For T13 and sex-chromosome anomalies, further prospective studies are necessary to confirm its utility.
Keywords: Aneuploidy; Down syndrome; Patau syndrome; Prenatal diagnosis; Trisomy 18.
Conflict of interest statement
Figures

References
-
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 88, December 2007. Invasive prenatal testing for aneuploidy. Obstet Gynecol. 2007;110:1459–1467. - PubMed
-
- Tabor A, Alfirevic Z. Update on procedure-related risks for prenatal diagnosis techniques. Fetal Diagn Ther. 2010;27:1–7. - PubMed
-
- Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005;353:2001–2011. - PubMed
-
- Nicolaides KH. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol. 2004;191:45–67. - PubMed
-
- Rozenberg P, Bussieres L, Chevret S, Bernard JP, Malagrida L, Cuckle H, et al. Screening for Down syndrome using first-trimester combined screening followed by second-trimester ultrasound examination in an unselected population. Am J Obstet Gynecol. 2006;195:1379–1387. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

