Experimental Models of Microvascular Immunopathology: The Example of Cerebral Malaria
- PMID: 26430675
- PMCID: PMC4586166
Experimental Models of Microvascular Immunopathology: The Example of Cerebral Malaria
Abstract
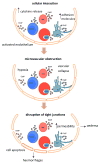
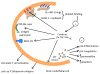
Human cerebral malaria is a severe and often lethal complication of Plasmodium falciparum infection. Complex host and parasite interactions should the precise mechanisms involved in the onset of this neuropathology. Adhesion of parasitised red blood cells and host cells to endothelial cells lead to profound endothelial alterations that trigger immunopathological changes, varying degrees of brain oedema and can compromise cerebral blood flow, cause cranial nerve dysfunction and hypoxia. Study of the cerebral pathology in human patients is limited to clinical and genetic field studies in endemic areas, thus cerebral malaria (CM) research relies heavily on experimental models. The availability of malaria models allows study from the inoculation of Plasmodium to the onset of disease and permit invasive experiments. Here, we discuss some aspects of our current understanding of CM, the experimental models available and some important recent findings extrapolated from these models.
Keywords: Cerebral malaria; Experimental malaria; Malaria; Plasmodium Immunopathology.
Conflict of interest statement
Authors declare no competing financial interests or conflict of interests.
Figures
References
-
- Mishra SK, Wiese L. Advances in the management of cerebral malaria in adults. Curr Opin Neurol. 2009;22:302–307. - PubMed
-
- Engwerda C, Belnoue E, Grüner AC, Rénia L. Experimental models of cerebral malaria. Curr Top Microbiol Immunol. 2005;297:103–143. - PubMed
-
- de Souza JB, Riley EM. Cerebral malaria: the contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect. 2002;4:291–300. - PubMed
-
- de Souza JB, Hafalla JC, Riley EM, Couper KN. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 2010;137:755–772. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
