A vesicular stomatitis virus glycoprotein epitope-incorporated oncolytic adenovirus overcomes CAR-dependency and shows markedly enhanced cancer cell killing and suppression of tumor growth
- PMID: 26430798
- PMCID: PMC4741496
- DOI: 10.18632/oncotarget.5332
A vesicular stomatitis virus glycoprotein epitope-incorporated oncolytic adenovirus overcomes CAR-dependency and shows markedly enhanced cancer cell killing and suppression of tumor growth
Abstract
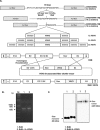
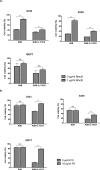
Utility of traditional oncolytic adenovirus (Ad) has been limited due to low expression of coxsackie and adenovirus receptor (CAR) in cancer cells which results in poor infectivity of Ads. Here with an aim of improving the efficiency of Ad's entry to the cell, we generated a novel tropism-expanded oncolytic Ad which contains the epitope of vesicular stomatitis virus glycoprotein (VSVG) at the HI-loop of Ad fiber. We generated 9 variants of oncolytic Ads with varying linkers and partial deletion to the fiber. Only one VSVG epitope-incorporated variant, RdB-1L-VSVG, which contains 1 linker and no deletion to fiber, was produced efficiently. Production of 3-dimensionaly stable fiber in RdB-1L-VSVG was confirmed by immunoblot analysis. RdB-1L-VSVG shows a remarkable improvement in cytotoxicity and total viral yield in cancer cells. RdB-1L-VSVG demonstrates enhanced cytotoxicity in cancer cells with subdued CAR-expression as it can be internalized by an alternate pathway. Competition assays with a CAR-specific antibody (Ab) or VSVG receptor, phosphatidyl serine (PS), reveals that cell internalization of RdB-1L-VSVG is mediated by both CAR and PS. Furthermore, treatment with RdB-1L-VSVG significantly enhanced anti-tumor effect in vivo. These studies demonstrate that the strategy to expand oncolytic Ad tropism may significantly improve therapeutic profile for cancer treatment.
Keywords: CAR dependency; VSVG; fiber; oncolytic adenovirus; therapeutic efficacy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. - PubMed
-
- Kirn D. Replication-selective oncolytic adenoviruses: virotherapy aimed at genetic targets in cancer. Oncogene. 2000;19:6660–6669. - PubMed
-
- Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of phase I and II trials. Expert Opin Biol Ther. 2001;1:525–538. - PubMed
-
- Nemunaitis J, Senzer N, Sarmiento S, Zhang YA, Arzaga R, Sands B, Maples P, Tong AW. A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer gene therapy. 2007;14:885–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

