Steroid Receptor Coactivator-3 (SRC-3/AIB1) as a Novel Therapeutic Target in Triple Negative Breast Cancer and Its Inhibition with a Phospho-Bufalin Prodrug
- PMID: 26431029
- PMCID: PMC4592245
- DOI: 10.1371/journal.pone.0140011
Steroid Receptor Coactivator-3 (SRC-3/AIB1) as a Novel Therapeutic Target in Triple Negative Breast Cancer and Its Inhibition with a Phospho-Bufalin Prodrug
Abstract
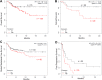
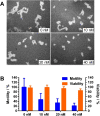
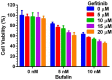
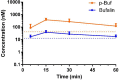
Triple negative breast cancer (TNBC) has the poorest prognosis of all types of breast cancer and currently lacks efficient targeted therapy. Chemotherapy is the traditional standard-of-care for TNBC, but is frequently accompanied by severe side effects. Despite the fact that high expression of steroid receptor coactivator 3 (SRC-3) is correlated with poor survival in estrogen receptor positive breast cancer patients, its role in TNBC has not been extensively investigated. Here, we show that high expression of SRC-3 correlates with both poor overall survival and post progression survival in TNBC patients, suggesting that SRC-3 can serve as a prognostic marker for TNBC. Furthermore, we demonstrated that bufalin, a SRC-3 small molecule inhibitor, when introduced even at nM concentrations, can significantly reduce TNBC cell viability and motility. However, because bufalin has minimal water solubility, its in vivo application is limited. Therefore, we developed a water soluble prodrug, 3-phospho-bufalin, to facilitate its in vivo administration. In addition, we demonstrated that 3-phospho-bufalin can effectively inhibit tumor growth in an orthotopic TNBC mouse model, suggesting its potential application as a targeted therapy for TNBC treatment.
Conflict of interest statement
Figures




Similar articles
-
Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1.Cancer Res. 2014 Mar 1;74(5):1506-1517. doi: 10.1158/0008-5472.CAN-13-2939. Epub 2014 Jan 3. Cancer Res. 2014. PMID: 24390736 Free PMC article.
-
miR-155-5p antagonizes the apoptotic effect of bufalin in triple-negative breast cancer cells.Anticancer Drugs. 2016 Jan;27(1):9-16. doi: 10.1097/CAD.0000000000000296. Anticancer Drugs. 2016. PMID: 26398931
-
Depletion of the Transcriptional Coactivator Amplified in Breast Cancer 1 (AIB1) Uncovers Functionally Distinct Subpopulations in Triple-Negative Breast Cancer.Neoplasia. 2019 Oct;21(10):963-973. doi: 10.1016/j.neo.2019.07.001. Epub 2019 Aug 19. Neoplasia. 2019. PMID: 31437536 Free PMC article.
-
Androgen Receptor Biology in Triple Negative Breast Cancer: a Case for Classification as AR+ or Quadruple Negative Disease.Horm Cancer. 2015 Dec;6(5-6):206-13. doi: 10.1007/s12672-015-0232-3. Epub 2015 Jul 23. Horm Cancer. 2015. PMID: 26201402 Free PMC article. Review.
-
SRC-3 has a role in cancer other than as a nuclear receptor coactivator.Int J Biol Sci. 2011;7(5):664-72. doi: 10.7150/ijbs.7.664. Epub 2011 May 24. Int J Biol Sci. 2011. PMID: 21647249 Free PMC article. Review.
Cited by
-
The pregnane X receptor (PXR) and the nuclear receptor corepressor 2 (NCoR2) modulate cell growth in head and neck squamous cell carcinoma.PLoS One. 2018 Feb 22;13(2):e0193242. doi: 10.1371/journal.pone.0193242. eCollection 2018. PLoS One. 2018. PMID: 29470550 Free PMC article.
-
Timing and Targeting of Treatment in Left Ventricular Hypertrophy.Methodist Debakey Cardiovasc J. 2017 Jan-Mar;13(1):9-14. doi: 10.14797/mdcj-13-1-9. Methodist Debakey Cardiovasc J. 2017. PMID: 28413576 Free PMC article. Review.
-
Lead Compound Development of SRC-3 Inhibitors with Improved Pharmacokinetic Properties and Anticancer Efficacy.J Med Chem. 2024 Apr 11;67(7):5333-5350. doi: 10.1021/acs.jmedchem.3c01596. Epub 2024 Mar 29. J Med Chem. 2024. PMID: 38551814 Free PMC article.
-
The Role of Steroid Receptor Coactivators in Hormone Dependent Cancers and Their Potential as Therapeutic Targets.Horm Cancer. 2016 Aug;7(4):229-35. doi: 10.1007/s12672-016-0261-6. Epub 2016 Apr 28. Horm Cancer. 2016. PMID: 27125199 Free PMC article. Review.
-
Molecular Pathways: Targeting Steroid Receptor Coactivators in Cancer.Clin Cancer Res. 2016 Nov 15;22(22):5403-5407. doi: 10.1158/1078-0432.CCR-15-1958. Epub 2016 Sep 21. Clin Cancer Res. 2016. PMID: 27654711 Free PMC article.
References
-
- Penault-Llorca F, Viale G (2012) Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann Oncol 23 Suppl 6: vi19–22. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

