Effects of Citalopram on Sutural and Calvarial Cell Processes
- PMID: 26431045
- PMCID: PMC4592261
- DOI: 10.1371/journal.pone.0139719
Effects of Citalopram on Sutural and Calvarial Cell Processes
Abstract
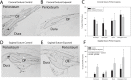
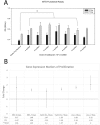
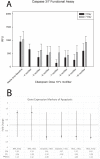
The use of selective serotonin reuptake inhibitors (SSRIs) for the treatment of depression during pregnancy is suggested to increase the incidence of craniofacial abnormalities including craniosynostosis. Little is known about this mechanism, however based on previous data we propose a mechanism that affects cell cycle. Excessive proliferation, and reduction in apoptosis may lead to hyperplasia within the suture that may allow for differentiation, bony infiltration, and fusion. Here we utilized in vivo and in vitro analysis to investigate this proposed phenomenon. For in vivo analysis we used C57BL-6 wild-type breeders treated with a clinical dose of citalopram during the third trimester of pregnancy to produce litters exposed to the SSRI citalopram in utero. At post-natal day 15 sutures were harvested from resulting pups and subjected to histomorphometric analysis for proliferation (PCNA) and apoptosis (TUNEL). For in vitro studies, we used mouse calvarial pre-osteoblast cells (MC3T3-E1) to assess proliferation (MTS), apoptosis (Caspase 3/7-activity), and gene expression after exposure to titrated doses of citalopram. In vivo analysis for PCNA suggested segregation of effect by location, with the sagittal suture, showing a statistically significant increase in proliferative response. The coronal suture was not similarly affected, however there was a decrease in apoptotic activity at the dural edge as compared to the periosteal edge. No differences in apoptosis by suture or area due to SSRI exposure were observed. In vitro results suggest citalopram exposure increased proliferation and proliferative gene expression, and decreased apoptosis of the MC3T3-E1 cells. Decreased apoptosis was not confirmed in vivo however, an increase in proliferation without a concomitant increase in apoptosis is still defined as hyperplasia. Thus prenatal SSRI exposure may exert a negative effect on post-natal growth through a hyperplasia effect at the cranial growth sites perhaps leading to clinically significant craniofacial abnormalities.
Conflict of interest statement
Figures

Similar articles
-
Selective serotonin reuptake inhibitor exposure alters osteoblast gene expression and craniofacial development in mice.Birth Defects Res A Clin Mol Teratol. 2014 Dec;100(12):912-23. doi: 10.1002/bdra.23323. Epub 2014 Oct 12. Birth Defects Res A Clin Mol Teratol. 2014. PMID: 25308507
-
Regional differentiation of cranial suture-associated dura mater in vivo and in vitro: implications for suture fusion and patency.J Bone Miner Res. 2000 Dec;15(12):2413-30. doi: 10.1359/jbmr.2000.15.12.2413. J Bone Miner Res. 2000. PMID: 11127206
-
Effects of In Utero Thyroxine Exposure on Murine Cranial Suture Growth.PLoS One. 2016 Dec 13;11(12):e0167805. doi: 10.1371/journal.pone.0167805. eCollection 2016. PLoS One. 2016. PMID: 27959899 Free PMC article.
-
Selective serotonin re-uptake inhibitors affect craniofacial structures in a mouse model.PLoS One. 2024 Jul 18;19(7):e0307134. doi: 10.1371/journal.pone.0307134. eCollection 2024. PLoS One. 2024. PMID: 39024220 Free PMC article.
-
Sutural biology and the correlates of craniosynostosis.Am J Med Genet. 1993 Oct 1;47(5):581-616. doi: 10.1002/ajmg.1320470507. Am J Med Genet. 1993. PMID: 8266985 Review.
Cited by
-
In utero exposure to electronic cigarette carriers alters craniofacial morphology.PLoS One. 2025 Jun 30;20(6):e0327190. doi: 10.1371/journal.pone.0327190. eCollection 2025. PLoS One. 2025. PMID: 40587499 Free PMC article.
-
Maternal exposure to SSRIs or SNRIs and the risk of congenital abnormalities in offspring: A systematic review and meta-analysis.PLoS One. 2023 Nov 29;18(11):e0294996. doi: 10.1371/journal.pone.0294996. eCollection 2023. PLoS One. 2023. PMID: 38019759 Free PMC article.
-
Gene/environment interactions in craniosynostosis: A brief review.Orthod Craniofac Res. 2017 Jun;20 Suppl 1(Suppl 1):8-11. doi: 10.1111/ocr.12153. Orthod Craniofac Res. 2017. PMID: 28643932 Free PMC article. Review.
-
Pharmacological exposures may precipitate craniosynostosis through targeted stem cell depletion.Stem Cell Res. 2019 Oct;40:101528. doi: 10.1016/j.scr.2019.101528. Epub 2019 Aug 6. Stem Cell Res. 2019. PMID: 31415959 Free PMC article.
-
Selective serotonin reuptake inhibitors (SSRI) affect murine bone lineage cells.Life Sci. 2020 Aug 15;255:117827. doi: 10.1016/j.lfs.2020.117827. Epub 2020 May 22. Life Sci. 2020. PMID: 32450170 Free PMC article.
References
-
- Dinlen N, Zenciroglu A, Dilli D, Aydin B, Beken S, Okumus N. Treacher Collins syndrome with multiple congenital heart defects after paroxetine exposure: case report. Genetic counseling. 2014;25(1):7–11. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

