Hyperglycemic Stress Impairs the Stemness Capacity of Kidney Stem Cells in Rats
- PMID: 26431335
- PMCID: PMC4592017
- DOI: 10.1371/journal.pone.0139607
Hyperglycemic Stress Impairs the Stemness Capacity of Kidney Stem Cells in Rats
Abstract
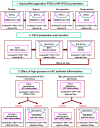
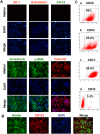
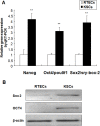
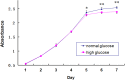
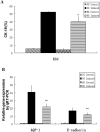
The incidence of acute kidney injury in patients with diabetes is significantly higher than that of patients without diabetes, and may be associated with the poor stemness capacity of kidney stem cells (KSCs) and limited recovery of injured renal tubules. To investigate the effects of hyperglycemic stress on KSC stemness, KSCs were isolated from the rat renal papilla and analyzed for their self-renewal and differentiation abilities. Our results showed that isolated KSCs expressed the mesenchymal stem cell markers N-cadherin, Nestin, CD133, CD29, CD90, and CD73. Moreover, KSCs co-cultured with hypoxia-injured renal tubular epithelial cell (RTECs) induced the expression of the mature epithelial cell marker CK18, suggesting that the KSCs could differentiate into RTECs in vitro. However, KSC proliferation, differentiation ability and tolerance to hypoxia were decreased in high-glucose cultures. Taken together, these results suggest the high-glucose microenvironment can damage the reparative ability of KSCs. It may result in a decreased of recovery capability of renal tubules from injury.
Conflict of interest statement
Figures


Similar articles
-
[Isolation and Identification of Rat Kidney Stem Cells].Sichuan Da Xue Xue Bao Yi Xue Ban. 2015 Sep;46(5):667-72. Sichuan Da Xue Xue Bao Yi Xue Ban. 2015. PMID: 26619532 Chinese.
-
[Induced differentiation of rat kidney stem cells into renal tubular epithelial cells].Nan Fang Yi Ke Da Xue Xue Bao. 2015 Feb;35(2):163-7. Nan Fang Yi Ke Da Xue Xue Bao. 2015. PMID: 25736106 Chinese.
-
[High glucose reduced the repair function of kidney stem cells conditional medium to the hypoxia-injured renal tubular epithelium cells].Beijing Da Xue Xue Bao Yi Xue Ban. 2017 Feb 18;49(1):125-30. Beijing Da Xue Xue Bao Yi Xue Ban. 2017. PMID: 28203018 Chinese.
-
[Effects of kidney stem cells on injure-recovery of human renal tubular epithelial cells].Beijing Da Xue Xue Bao Yi Xue Ban. 2013 Aug 18;45(4):619-24. Beijing Da Xue Xue Bao Yi Xue Ban. 2013. PMID: 23939175 Chinese.
-
Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells.Int J Mol Med. 2008 Sep;22(3):325-32. Int J Mol Med. 2008. PMID: 18698491
Cited by
-
Exogenous pericyte delivery protects the mouse kidney from chronic ischemic injury.Am J Physiol Renal Physiol. 2022 Nov 1;323(5):F527-F538. doi: 10.1152/ajprenal.00064.2022. Epub 2022 Sep 1. Am J Physiol Renal Physiol. 2022. PMID: 36049063 Free PMC article.
-
Renovascular disease induces mitochondrial damage in swine scattered tubular cells.Am J Physiol Renal Physiol. 2019 Nov 1;317(5):F1142-F1153. doi: 10.1152/ajprenal.00276.2019. Epub 2019 Aug 28. Am J Physiol Renal Physiol. 2019. PMID: 31461348 Free PMC article.
-
Renal Artery Stenosis Alters Gene Expression in Swine Scattered Tubular-Like Cells.Int J Mol Sci. 2019 Oct 12;20(20):5069. doi: 10.3390/ijms20205069. Int J Mol Sci. 2019. PMID: 31614781 Free PMC article.
-
High CD133 expression in proximal tubular cells in diabetic kidney disease: good or bad?J Transl Med. 2024 Feb 16;22(1):159. doi: 10.1186/s12967-024-04950-0. J Transl Med. 2024. PMID: 38365731 Free PMC article.
-
Renal Ischemia Induces Epigenetic Changes in Apoptotic, Proteolytic, and Mitochondrial Genes in Swine Scattered Tubular-like Cells.Cells. 2022 May 31;11(11):1803. doi: 10.3390/cells11111803. Cells. 2022. PMID: 35681498 Free PMC article.
References
-
- Liu Y, Ma Q, Yang G, Zhao J, Liu S, Ao Q, et al. Early renal tubulointerstitial changes and their interventions with Shenkang Injection in diabetic rats. Zhonghua Yi Xue Za Zhi. 2015;95(4):289–293. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

