Development of Physiologically Based Pharmacokinetic/Pharmacodynamic Model for Indomethacin Disposition in Pregnancy
- PMID: 26431339
- PMCID: PMC4592215
- DOI: 10.1371/journal.pone.0139762
Development of Physiologically Based Pharmacokinetic/Pharmacodynamic Model for Indomethacin Disposition in Pregnancy
Abstract
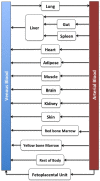
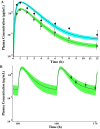
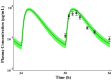
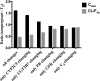
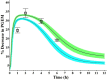
Findings of a recent clinical study showed indomethacin has lower plasma levels and higher steady-state apparent clearance in pregnant subjects when compared to those in non-pregnant subjects reported in separate studies. Thus, in the current work we developed a pregnancy physiological based pharmacokinetic/pharmacodynamic (PBPK/PD) model for indomethacin to explain the differences in indomethacin pharmacokinetics between pregnancy and non-pregnancy. A whole-body PBPK model with key pregnancy-related physiological changes was developed to characterize indomethacin PK in pregnant women and compare these parameters to those in non-pregnant subjects. Data related to maternal physiological and biological changes were obtained from literature and incorporated into the structural PBPK model that describes non-pregnant PK data. Changes in indomethacin area under the curve (AUC), maximum concentration (Cmax) and average steady-state concentration (Cave) in pregnant women were predicted. Model-simulated PK profiles were in agreement with observed data. The predicted mean ratio (non-pregnant:second trimester (T2)) of indomethacin Cave was 1.6 compared to the observed value of 1.59. In addition, the predicted steady-state apparent clearance (CL/Fss) ratio was almost similar to the observed value (0.46 vs. 0.42). Sensitivity analysis suggested changes in CYP2C9 activity, and to a lesser extent UGT2B7, as the primary factor contributing to differences in indomethacin disposition between pregnancy and non-pregnancy. The developed PBPK model which integrates prior physiological knowledge, in vitro and in vivo data, allowed the successful prediction of indomethacin disposition during T2. Our PBPK/PD model suggested a higher indomethacin dosing requirement during pregnancy.
Conflict of interest statement
Figures
Similar articles
-
Physiologically-based pharmacokinetic modeling of renally excreted antiretroviral drugs in pregnant women.Br J Clin Pharmacol. 2015 Nov;80(5):1031-41. doi: 10.1111/bcp.12685. Epub 2015 Jul 22. Br J Clin Pharmacol. 2015. PMID: 26011128 Free PMC article.
-
Expansion of a PBPK model to predict disposition in pregnant women of drugs cleared via multiple CYP enzymes, including CYP2B6, CYP2C9 and CYP2C19.Br J Clin Pharmacol. 2014 Mar;77(3):554-70. doi: 10.1111/bcp.12207. Br J Clin Pharmacol. 2014. PMID: 23834474 Free PMC article.
-
Prediction of maternal and fetal pharmacokinetics of indomethacin in pregnancy.Br J Clin Pharmacol. 2022 Jan;88(1):271-281. doi: 10.1111/bcp.14960. Epub 2021 Jul 22. Br J Clin Pharmacol. 2022. PMID: 34185331
-
The absorption, distribution, metabolism and elimination characteristics of small interfering RNA therapeutics and the opportunity to predict disposition in pregnant women.Drug Metab Dispos. 2025 Jan;53(1):100018. doi: 10.1124/dmd.123.001383. Epub 2024 Nov 22. Drug Metab Dispos. 2025. PMID: 39884813 Review.
-
PK-PD integration and PK-PD modelling of nonsteroidal anti-inflammatory drugs: principles and applications in veterinary pharmacology.J Vet Pharmacol Ther. 2004 Dec;27(6):491-502. doi: 10.1111/j.1365-2885.2004.00618.x. J Vet Pharmacol Ther. 2004. PMID: 15601443 Review.
Cited by
-
Application of a Physiologically Based Pharmacokinetic Model to Develop a Veterinary Amorphous Enrofloxacin Solid Dispersion.Pharmaceutics. 2021 Apr 22;13(5):602. doi: 10.3390/pharmaceutics13050602. Pharmaceutics. 2021. PMID: 33922109 Free PMC article.
-
Effect of CYP2C9 Polymorphisms on the Pharmacokinetics of Indomethacin During Pregnancy.Eur J Drug Metab Pharmacokinet. 2019 Feb;44(1):83-89. doi: 10.1007/s13318-018-0505-7. Eur J Drug Metab Pharmacokinet. 2019. PMID: 30159654 Free PMC article.
-
Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling to Predict the Impact of CYP2C9 Genetic Polymorphisms, Co-Medication and Formulation on the Pharmacokinetics and Pharmacodynamics of Flurbiprofen.Pharmaceutics. 2020 Nov 2;12(11):1049. doi: 10.3390/pharmaceutics12111049. Pharmaceutics. 2020. PMID: 33147873 Free PMC article.
-
A Physiologically-Based Pharmacokinetic Model to Predict Human Fetal Exposure for a Drug Metabolized by Several CYP450 Pathways.Clin Pharmacokinet. 2017 May;56(5):537-550. doi: 10.1007/s40262-016-0457-5. Clin Pharmacokinet. 2017. PMID: 27766562
-
Indomethacin Increases the Efficacy of Oxygen Utilization of Colonic Mitochondria and Uncouples Hepatic Mitochondria in Tissue Homogenates From Healthy Rats.Front Med (Lausanne). 2020 Aug 21;7:463. doi: 10.3389/fmed.2020.00463. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32974368 Free PMC article.
References
-
- Verbeeck RK, Blackburn JL, Loewen GR. Clinical pharmacokinetics of non-steroidal anti-inflammatory drugs. Clinical pharmacokinetics. 1983;8(4):297–331. . - PubMed
-
- Zuckerman H, Shalev E, Gilad G, Katzuni E. Further study of the inhibition of premature labor by indomethacin. Part I. Journal of perinatal medicine. 1984;12(1):19–23. . - PubMed
-
- Zuckerman H, Shalev E, Gilad G, Katzuni E. Further study of the inhibition of premature labor by indomethacin. Part II double-blind study. Journal of perinatal medicine. 1984;12(1):25–9. . - PubMed
-
- Rane A, Oelz O, Frolich JC, Seyberth HW, Sweetman BJ, Watson JT, et al. Relation between plasma concentration of indomethacin and its effect on prostaglandin synthesis and platelet aggregation in man. Clinical pharmacology and therapeutics. 1978;23(6):658–68. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

