Sympathetic Neurotransmitters Modulate Osteoclastogenesis and Osteoclast Activity in the Context of Collagen-Induced Arthritis
- PMID: 26431344
- PMCID: PMC4592252
- DOI: 10.1371/journal.pone.0139726
Sympathetic Neurotransmitters Modulate Osteoclastogenesis and Osteoclast Activity in the Context of Collagen-Induced Arthritis
Abstract
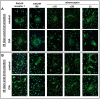
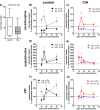
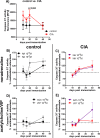
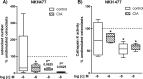
Excessive synovial osteoclastogenesis is a hallmark of rheumatoid arthritis (RA). Concomitantly, local synovial changes comprise neuronal components of the peripheral sympathetic nervous system. Here, we wanted to analyze if collagen-induced arthritis (CIA) alters bone marrow-derived macrophage (BMM) osteoclastogenesis and osteoclast activity, and how sympathetic neurotransmitters participate in this process. Therefore, BMMs from Dark Agouti rats at different CIA stages were differentiated into osteoclasts in vitro and osteoclast number, cathepsin K activity, matrix resorption and apoptosis were analyzed in the presence of acetylcholine (ACh), noradrenaline (NA) vasoactive intestinal peptide (VIP) and assay-dependent, adenylyl cyclase activator NKH477. We observed modulation of neurotransmitter receptor mRNA expression in CIA osteoclasts without affecting protein level. CIA stage-dependently altered marker gene expression associated with osteoclast differentiation and activity without affecting osteoclast number or activity. Neurotransmitter stimulation modulated osteoclast differentiation, apoptosis and activity. VIP, NA and adenylyl cyclase activator NKH477 inhibited cathepsin K activity and osteoclastogenesis (NKH477, 10(-6) M NA) whereas ACh mostly acted pro-osteoclastogenic. We conclude that CIA alone does not affect metabolism of in vitro generated osteoclasts whereas stimulation with NA, VIP plus specific activation of adenylyl cyclase induced anti-resorptive effects probably mediated via cAMP signaling. Contrary, we suggest pro-osteoclastogenic and pro-resorptive properties of ACh mediated via muscarinic receptors.
Conflict of interest statement
Figures

Similar articles
-
Reactivity of rat bone marrow-derived macrophages to neurotransmitter stimulation in the context of collagen II-induced arthritis.Arthritis Res Ther. 2015 Jun 24;17(1):169. doi: 10.1186/s13075-015-0684-4. Arthritis Res Ther. 2015. PMID: 26104678 Free PMC article.
-
Analysis of the kinetics of osteoclastogenesis in arthritic rats.Arthritis Rheum. 2005 Oct;52(10):3192-201. doi: 10.1002/art.21343. Arthritis Rheum. 2005. PMID: 16200623
-
BSP and RANKL induce osteoclastogenesis and bone resorption synergistically.J Bone Miner Res. 2005 Sep;20(9):1669-79. doi: 10.1359/JBMR.050511. Epub 2005 May 16. J Bone Miner Res. 2005. PMID: 16059638
-
Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis.Am J Pathol. 2002 Oct;161(4):1419-27. doi: 10.1016/S0002-9440(10)64417-3. Am J Pathol. 2002. PMID: 12368214 Free PMC article.
-
Gene expression relevant to osteoclastogenesis in the synovium and bone marrow of mature rats with collagen-induced arthritis.Rheumatology (Oxford). 2004 Dec;43(12):1496-503. doi: 10.1093/rheumatology/keh395. Epub 2004 Sep 7. Rheumatology (Oxford). 2004. PMID: 15353610
Cited by
-
[New insights into the function of bone marrow].Z Rheumatol. 2018 May;77(Suppl 1):4-7. doi: 10.1007/s00393-018-0456-z. Z Rheumatol. 2018. PMID: 29691692 Review. German. No abstract available.
-
Astragaloside regulates lncRNA LOC100912373 and the miR‑17‑5p/PDK1 axis to inhibit the proliferation of fibroblast‑like synoviocytes in rats with rheumatoid arthritis.Int J Mol Med. 2021 Jul;48(1):130. doi: 10.3892/ijmm.2021.4963. Epub 2021 May 20. Int J Mol Med. 2021. PMID: 34013364 Free PMC article.
-
A Clinical Approach for the Use of VIP Axis in Inflammatory and Autoimmune Diseases.Int J Mol Sci. 2019 Dec 20;21(1):65. doi: 10.3390/ijms21010065. Int J Mol Sci. 2019. PMID: 31861827 Free PMC article. Review.
-
The Neuropeptide VIP Limits Human Osteoclastogenesis: Clinical Associations with Bone Metabolism Markers in Patients with Early Arthritis.Biomedicines. 2021 Dec 10;9(12):1880. doi: 10.3390/biomedicines9121880. Biomedicines. 2021. PMID: 34944693 Free PMC article.
-
Impact of the Autonomic Nervous System on the Skeleton.Physiol Rev. 2018 Jul 1;98(3):1083-1112. doi: 10.1152/physrev.00014.2017. Physiol Rev. 2018. PMID: 29717928 Free PMC article. Review.
References
-
- Yamazaki H, Kunisada T, Yamane T, Hayashi SI. Presence of osteoclast precursors in colonies cloned in the presence of hematopoietic colony-stimulating factors. Experimental hematology. 2001;29(1):68–76. Epub 2001/02/13. . - PubMed
-
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. Epub 1998/05/06. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

