Vesicular stomatitis virus expressing interferon-β is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer
- PMID: 26431376
- PMCID: PMC4741756
- DOI: 10.18632/oncotarget.5320
Vesicular stomatitis virus expressing interferon-β is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer
Abstract
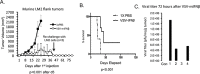
Vesicular stomatitis virus (VSV) is a potent oncolytic virus for many tumors. VSV that produces interferon-β (VSV-IFNβ) is now in early clinical testing for solid tumors. Here, the preclinical activity of VSV and VSV-IFNβ against non-small cell lung cancer (NSCLC) is reported. NSCLC cell lines were treated in vitro with VSV expressing green fluorescence protein (VSV-GFP) and VSV-IFNβ. VSV-GFP and VSV-IFNβ were active against NSCLC cells. JAK/STAT inhibition with ruxolitinib re-sensitized resistant H838 cells to VSV-IFNβ mediated oncolysis. Intratumoral injections of VSV-GFP and VSV-IFNβ reduced tumor growth and weight in H2009 nude mouse xenografts (p < 0.01). A similar trend was observed in A549 xenografts. Syngeneic LM2 lung tumors grown in flanks of A/J mice were injected with VSV-IFNβ intratumorally. Treatment of LM2 tumors with VSV-IFNβ resulted in tumor regression, prolonged survival (p < 0.0001), and cure of 30% of mice. Intratumoral injection of VSV-IFNβ resulted in decreased tumor-infiltrating regulatory T cells (Treg) and increased CD8+ T cells. Tumor cell expression of PDL-1 was increased after VSV-IFNβ treatment. VSV-IFNβ has potent antitumor effects and promotes systemic antitumor immunity. These data support further clinical investigation of VSV-IFNβ for NSCLC.
Keywords: NSCLC; Treg; VSV; interferon-β; oncolytic virus.
Conflict of interest statement
The authors have no relevant conflicts of interest to disclose.
Figures





Similar articles
-
JAK/STAT inhibition with ruxolitinib enhances oncolytic virotherapy in non-small cell lung cancer models.Cancer Gene Ther. 2019 Nov;26(11-12):411-418. doi: 10.1038/s41417-018-0074-6. Epub 2019 Jan 9. Cancer Gene Ther. 2019. PMID: 30622322
-
Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-β.Cancer Gene Ther. 2012 Jul;19(7):443-50. doi: 10.1038/cgt.2012.14. Epub 2012 Apr 20. Cancer Gene Ther. 2012. PMID: 22522623 Free PMC article.
-
Oncolytic VSV Primes Differential Responses to Immuno-oncology Therapy.Mol Ther. 2017 Aug 2;25(8):1917-1932. doi: 10.1016/j.ymthe.2017.05.006. Epub 2017 Jun 2. Mol Ther. 2017. PMID: 28578991 Free PMC article.
-
Preclinical efficacy of oncolytic VSV-IFNβ in treating cancer: A systematic review.Front Immunol. 2023 Mar 31;14:1085940. doi: 10.3389/fimmu.2023.1085940. eCollection 2023. Front Immunol. 2023. PMID: 37063914 Free PMC article.
-
Immunotherapy for thoracic oncology gone viral.Immunotherapy. 2018 Apr;10(5):383-390. doi: 10.2217/imt-2017-0148. Immunotherapy. 2018. PMID: 29473469 Review.
Cited by
-
Metabolic barriers to cancer immunotherapy.Nat Rev Immunol. 2021 Dec;21(12):785-797. doi: 10.1038/s41577-021-00541-y. Epub 2021 Apr 29. Nat Rev Immunol. 2021. PMID: 33927375 Free PMC article. Review.
-
Trial Watch: Oncolytic viro-immunotherapy of hematologic and solid tumors.Oncoimmunology. 2018 Aug 27;7(12):e1503032. doi: 10.1080/2162402X.2018.1503032. eCollection 2018. Oncoimmunology. 2018. PMID: 30524901 Free PMC article. Review.
-
Engaging Pattern Recognition Receptors in Solid Tumors to Generate Systemic Antitumor Immunity.Cancer Treat Res. 2022;183:91-129. doi: 10.1007/978-3-030-96376-7_3. Cancer Treat Res. 2022. PMID: 35551657
-
Oncolytic Virus-Based Cytokine Expression to Improve Immune Activity in Brain and Solid Tumors.Mol Ther Oncolytics. 2019 Mar 20;13:14-21. doi: 10.1016/j.omto.2019.03.001. eCollection 2019 Jun 28. Mol Ther Oncolytics. 2019. PMID: 30997392 Free PMC article. Review.
-
Taking a Stab at Cancer; Oncolytic Virus-Mediated Anti-Cancer Vaccination Strategies.Biomedicines. 2017 Jan 4;5(1):3. doi: 10.3390/biomedicines5010003. Biomedicines. 2017. PMID: 28536346 Free PMC article. Review.
References
-
- Patel MR, Kratzke RA. Oncolytic virus therapy for cancer: the first wave of translational clinical trials. Transl Res. 2013;161:355–364. - PubMed
-
- Li Q, Wei YQ, Wen YJ, Zhao X, Tian L, Yang L, Mao YQ, Kan B, Wu Y, Ding ZY, Deng HX, Li J, Luo Y, et al. Induction of apoptosis and tumor regression by vesicular stomatitis virus in the presence of gemcitabine in lung cancer. Int J Cancer. 2004;112:143–149. - PubMed
-
- Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. - PubMed
-
- Willmon CL, Saloura V, Fridlender ZG, Wongthida P, Diaz RM, Thompson J, Kottke T, Federspiel M, Barber G, Albelda SM, Vile RG. Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer research. 2009;69:7713–7720. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

