Inhibition of testicular embryonal carcinoma cell tumorigenicity by peroxisome proliferator-activated receptor-β/δ- and retinoic acid receptor-dependent mechanisms
- PMID: 26431381
- PMCID: PMC4742180
- DOI: 10.18632/oncotarget.5415
Inhibition of testicular embryonal carcinoma cell tumorigenicity by peroxisome proliferator-activated receptor-β/δ- and retinoic acid receptor-dependent mechanisms
Abstract
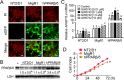
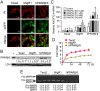
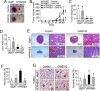
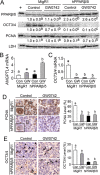
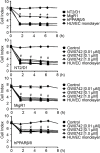
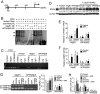
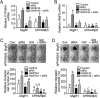
Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) has important physiological functions in control of cell growth, lipid and glucose homeostasis, differentiation and inflammation. To investigate the role of PPARβ/δ in cancer, stable human testicular embryonal carcinoma cell lines were developed that constitutively express PPARβ/δ. Expression of PPARβ/δ caused enhanced activation of the receptor, and this significantly decreased proliferation, migration, invasion, anchorage-independent growth, and also reduced tumor mass and volume of ectopic xenografts derived from NT2/D1 cells compared to controls. The changes observed in xenografts were associated with decreased PPARβ/δ-dependent expression of proliferating cell nuclear antigen and octamer-binding transcription factor-3/4, suggesting suppressed tumor proliferation and induction of differentiation. Inhibition of migration and invasion was mediated by PPARβ/δ competing with formation of the retinoic acid receptor (RAR)/retinoid X receptor (RXR) complex, resulting in attenuation of RARα-dependent matrix metalloproteinase-2 expression and activity. These results demonstrate that PPARβ/δ mediates attenuation of human testicular embryonal carcinoma cell progression through a novel RAR-dependent mechanism and suggest that activation of PPARβ/δ inhibits RAR/RXR dimerization and represents a new therapeutic strategy.
Keywords: matrix metalloproteinase-2; peroxisome proliferator-activated receptor-β/δ; retinoic acid receptor; testicular embryonal carcinoma; tumorigenicity.
Conflict of interest statement
All authors have no perceived conflicts of interest to disclose.
Figures




Similar articles
-
Peroxisome proliferator-activated receptor-β/δ inhibits human neuroblastoma cell tumorigenesis by inducing p53- and SOX2-mediated cell differentiation.Mol Carcinog. 2017 May;56(5):1472-1483. doi: 10.1002/mc.22607. Epub 2017 Jan 13. Mol Carcinog. 2017. PMID: 27996177 Free PMC article.
-
Stable over-expression of PPARβ/δ and PPARγ to examine receptor signaling in human HaCaT keratinocytes.Cell Signal. 2011 Dec;23(12):2039-50. doi: 10.1016/j.cellsig.2011.07.020. Epub 2011 Aug 4. Cell Signal. 2011. PMID: 21843636 Free PMC article.
-
Ligand activation of peroxisome proliferator-activated receptor-beta/delta inhibits cell proliferation in human HaCaT keratinocytes.Mol Pharmacol. 2008 Nov;74(5):1429-42. doi: 10.1124/mol.108.050609. Epub 2008 Aug 7. Mol Pharmacol. 2008. PMID: 18687807 Free PMC article.
-
Regulatory mechanisms mediated by peroxisome proliferator-activated receptor-β/δ in skin cancer.Mol Carcinog. 2019 Sep;58(9):1612-1622. doi: 10.1002/mc.23033. Epub 2019 May 6. Mol Carcinog. 2019. PMID: 31062422 Free PMC article. Review.
-
Nutritional strategies to modulate inflammation pathways via regulation of peroxisome proliferator-activated receptor β/δ.Nutr Rev. 2020 Mar 1;78(3):207-214. doi: 10.1093/nutrit/nuz058. Nutr Rev. 2020. PMID: 31584650 Review.
Cited by
-
PPAR Beta/Delta and the Hallmarks of Cancer.Cells. 2020 May 4;9(5):1133. doi: 10.3390/cells9051133. Cells. 2020. PMID: 32375405 Free PMC article. Review.
-
Inhibition of tumorigenesis by peroxisome proliferator-activated receptor (PPAR)-dependent cell cycle blocks in human skin carcinoma cells.Toxicology. 2018 Jul 1;404-405:25-32. doi: 10.1016/j.tox.2018.05.003. Epub 2018 May 3. Toxicology. 2018. PMID: 29729928 Free PMC article.
-
Editor's Highlight: PPARβ/δ and PPARγ Inhibit Melanoma Tumorigenicity by Modulating Inflammation and Apoptosis.Toxicol Sci. 2017 Oct 1;159(2):436-448. doi: 10.1093/toxsci/kfx147. Toxicol Sci. 2017. PMID: 28962521 Free PMC article.
-
Peroxisome proliferator-activated receptor-β/δ modulates mast cell phenotype.Immunology. 2017 Apr;150(4):456-467. doi: 10.1111/imm.12699. Epub 2017 Jan 24. Immunology. 2017. PMID: 27935639 Free PMC article.
-
Co-expression of carcinoembryonic antigen-related cell adhesion molecule 6 and 8 inhibits proliferation and invasiveness of breast carcinoma cells.Clin Exp Metastasis. 2019 Oct;36(5):423-432. doi: 10.1007/s10585-019-09981-2. Epub 2019 Jun 20. Clin Exp Metastasis. 2019. PMID: 31222613
References
-
- Voutsadakis IA. The chemosensitivity of testicular germ cell tumors. Cellular oncology. 2014;37:79–94. - PubMed
-
- Boublikova L, Buchler T, Stary J, Abrahamova J, Trka J. Molecular biology of testicular germ cell tumors: unique features awaiting clinical application. Critical reviews in oncology/hematology. 2014;89:366–385. - PubMed
-
- Bahrami A, Ro JY, Ayala AG. An overview of testicular germ cell tumors. Archives of pathology & laboratory medicine. 2007;131:1267–1280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

