Endocytosis‒Mediated Invasion and Pathogenicity of Streptococcus agalactiae in Rat Cardiomyocyte (H9C2)
- PMID: 26431539
- PMCID: PMC4592223
- DOI: 10.1371/journal.pone.0139733
Endocytosis‒Mediated Invasion and Pathogenicity of Streptococcus agalactiae in Rat Cardiomyocyte (H9C2)
Abstract
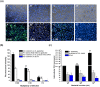
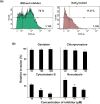
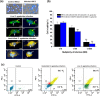
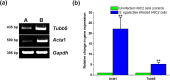
Streptococcus agalactiae infection causes high mortality in cardiovascular disease (CVD) patients, especially in case of setting prosthetic valve during cardiac surgery. However, the pathogenesis mechanism of S. agalactiae associate with CVD has not been well studied. Here, we have demonstrated the pathogenicity of S. agalactiae in rat cardiomyocytes (H9C2). Interestingly, both live and dead cells of S. agalactiae were uptaken by H9C2 cells. To further dissect the process of S. agalactiae internalization, we chemically inhibited discrete parts of cellular uptake system in H9C2 cells using genistein, chlorpromazine, nocodazole and cytochalasin B. Chemical inhibition of microtubule and actin formation by nocodazole and cytochalasin B impaired S. agalactiae internalization into H9C2 cells. Consistently, reverse‒ transcription PCR (RT‒PCR) and quantitative real time‒PCR (RT-qPCR) analyses also detected higher levels of transcripts for cytoskeleton forming genes, Acta1 and Tubb5 in S. agalactiae‒infected H9C2 cells, suggesting the requirement of functional cytoskeleton in pathogenesis. Host survival assay demonstrated that S. agalactiae internalization induced cytotoxicity in H9C2 cells. S. agalactiae cells grown with benzyl penicillin reduced its ability to internalize and induce cytotoxicity in H9C2 cells, which could be attributed with the removal of surface lipoteichoic acid (LTA) from S. agalactiae. Further, the LTA extracted from S. agalactiae also exhibited dose‒dependent cytotoxicity in H9C2 cells. Taken together, our data suggest that S. agalactiae cells internalized H9C2 cells through energy‒dependent endocytic processes and the LTA of S. agalactiae play major role in host cell internalization and cytotoxicity induction.
Conflict of interest statement
Figures
Similar articles
-
Teichoic acids of Streptococcus agalactiae: chemistry, cytotoxicity, and effect on bacterial adherence to human cells in tissue culture.Infect Immun. 1984 Feb;43(2):670-7. doi: 10.1128/iai.43.2.670-677.1984. Infect Immun. 1984. PMID: 6363297 Free PMC article.
-
Attenuated virulence of Streptococcus agalactiae deficient in D-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells.Mol Microbiol. 2003 Sep;49(6):1615-25. doi: 10.1046/j.1365-2958.2003.03655.x. Mol Microbiol. 2003. PMID: 12950925
-
RovS and its associated signaling peptide form a cell-to-cell communication system required for Streptococcus agalactiae pathogenesis.mBio. 2015 Jan 20;6(1):e02306-14. doi: 10.1128/mBio.02306-14. mBio. 2015. PMID: 25604789 Free PMC article.
-
[Lipoteichoic and teichoic acids of pathogenic streptococci: structure, functions, and role in interaction of the infectious agent with organism].Zh Mikrobiol Epidemiol Immunobiol. 2007 Nov-Dec;(6):100-7. Zh Mikrobiol Epidemiol Immunobiol. 2007. PMID: 18277547 Review. Russian.
-
Streptococcal toxins: role in pathogenesis and disease.Cell Microbiol. 2015 Dec;17(12):1721-41. doi: 10.1111/cmi.12531. Epub 2015 Nov 17. Cell Microbiol. 2015. PMID: 26433203 Review.
Cited by
-
Pannexin-1 regulation of ATP release promotes the invasion of pituitary adenoma.J Endocrinol Invest. 2025 Feb;48(2):317-332. doi: 10.1007/s40618-024-02445-9. Epub 2024 Nov 11. J Endocrinol Invest. 2025. PMID: 39527372
-
Targeting translocator protein protects against myocardial ischemia/reperfusion injury by alleviating mitochondrial dysfunction.Exp Ther Med. 2024 Jul 4;28(3):349. doi: 10.3892/etm.2024.12638. eCollection 2024 Sep. Exp Ther Med. 2024. PMID: 39071907 Free PMC article.
-
Trichosanthis Pericarpium Aqueous Extract Protects H9c2 Cardiomyocytes from Hypoxia/Reoxygenation Injury by Regulating PI3K/Akt/NO Pathway.Molecules. 2018 Sep 20;23(10):2409. doi: 10.3390/molecules23102409. Molecules. 2018. PMID: 30241309 Free PMC article.
-
TUBB Variants Underlying Different Phenotypes Result in Altered Vesicle Trafficking and Microtubule Dynamics.Int J Mol Sci. 2020 Feb 18;21(4):1385. doi: 10.3390/ijms21041385. Int J Mol Sci. 2020. PMID: 32085672 Free PMC article.
-
scDual-Seq of Toxoplasma gondii-infected mouse BMDCs reveals heterogeneity and differential infection dynamics.Front Immunol. 2023 Jul 27;14:1224591. doi: 10.3389/fimmu.2023.1224591. eCollection 2023. Front Immunol. 2023. PMID: 37575232 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

