Definitive Characterization of CA 19-9 in Resectable Pancreatic Cancer Using a Reference Set of Serum and Plasma Specimens
- PMID: 26431551
- PMCID: PMC4592020
- DOI: 10.1371/journal.pone.0139049
Definitive Characterization of CA 19-9 in Resectable Pancreatic Cancer Using a Reference Set of Serum and Plasma Specimens
Abstract
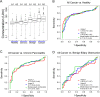
The validation of candidate biomarkers often is hampered by the lack of a reliable means of assessing and comparing performance. We present here a reference set of serum and plasma samples to facilitate the validation of biomarkers for resectable pancreatic cancer. The reference set includes a large cohort of stage I-II pancreatic cancer patients, recruited from 5 different institutions, and relevant control groups. We characterized the performance of the current best serological biomarker for pancreatic cancer, CA 19-9, using plasma samples from the reference set to provide a benchmark for future biomarker studies and to further our knowledge of CA 19-9 in early-stage pancreatic cancer and the control groups. CA 19-9 distinguished pancreatic cancers from the healthy and chronic pancreatitis groups with an average sensitivity and specificity of 70-74%, similar to previous studies using all stages of pancreatic cancer. Chronic pancreatitis patients did not show CA 19-9 elevations, but patients with benign biliary obstruction had elevations nearly as high as the cancer patients. We gained additional information about the biomarker by comparing two distinct assays. The two CA 9-9 assays agreed well in overall performance but diverged in measurements of individual samples, potentially due to subtle differences in antibody specificity as revealed by glycan array analysis. Thus, the reference set promises be a valuable resource for biomarker validation and comparison, and the CA 19-9 data presented here will be useful for benchmarking and for exploring relationships to CA 19-9.
Conflict of interest statement
Figures



Similar articles
-
A Plasma Biomarker Panel to Identify Surgically Resectable Early-Stage Pancreatic Cancer.J Natl Cancer Inst. 2017 Aug 1;109(8):djw341. doi: 10.1093/jnci/djw341. J Natl Cancer Inst. 2017. PMID: 28376184 Free PMC article.
-
MicroRNA biomarkers in whole blood for detection of pancreatic cancer.JAMA. 2014 Jan 22-29;311(4):392-404. doi: 10.1001/jama.2013.284664. JAMA. 2014. PMID: 24449318
-
Improved Pancreatic Adenocarcinoma Diagnosis in Jaundiced and Non-Jaundiced Pancreatic Adenocarcinoma Patients through the Combination of Routine Clinical Markers Associated to Pancreatic Adenocarcinoma Pathophysiology.PLoS One. 2016 Jan 25;11(1):e0147214. doi: 10.1371/journal.pone.0147214. eCollection 2016. PLoS One. 2016. PMID: 26808421 Free PMC article.
-
[Adenocarcinoma of the pancreas: are CA 19-9 assays useful?].Presse Med. 2008 Jan;37(1 Pt 2):88-94. doi: 10.1016/j.lpm.2007.05.029. Epub 2007 Nov 5. Presse Med. 2008. PMID: 17980545 Review. French.
-
Carbohydrate antigen 19-9 for differential diagnosis of pancreatic carcinoma and chronic pancreatitis.World J Gastroenterol. 2015 Apr 14;21(14):4323-33. doi: 10.3748/wjg.v21.i14.4323. World J Gastroenterol. 2015. PMID: 25892884 Free PMC article. Review.
Cited by
-
Evaluating Metabolite-Based Biomarkers for Early Diagnosis of Pancreatic Cancer: A Systematic Review.Metabolites. 2023 Jul 22;13(7):872. doi: 10.3390/metabo13070872. Metabolites. 2023. PMID: 37512579 Free PMC article. Review.
-
Glycans related to the CA19-9 antigen are elevated in distinct subsets of pancreatic cancers and improve diagnostic accuracy over CA19-9.Cell Mol Gastroenterol Hepatol. 2016 Feb 1;2(2):201-221.e15. doi: 10.1016/j.jcmgh.2015.12.003. Cell Mol Gastroenterol Hepatol. 2016. PMID: 26998508 Free PMC article.
-
Circulating Neoplastic-Immune Hybrid Cells Are Biomarkers of Occult Metastasis and Treatment Response in Pancreatic Cancer.Cancers (Basel). 2024 Oct 29;16(21):3650. doi: 10.3390/cancers16213650. Cancers (Basel). 2024. PMID: 39518088 Free PMC article.
-
Protein biomarkers and alternatively methylated cell-free DNA detect early stage pancreatic cancer.Gut. 2024 Mar 7;73(4):639-648. doi: 10.1136/gutjnl-2023-331074. Gut. 2024. PMID: 38123998 Free PMC article.
-
Pre-operative radiomics model for prognostication in resectable pancreatic adenocarcinoma with external validation.Eur Radiol. 2022 Apr;32(4):2492-2505. doi: 10.1007/s00330-021-08314-w. Epub 2021 Nov 10. Eur Radiol. 2022. PMID: 34757450
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

