Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes
- PMID: 26431796
- PMCID: PMC4651811
- DOI: 10.1016/j.taap.2015.09.022
Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes
Abstract
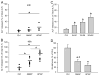
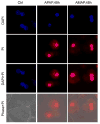
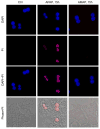
3'-Hydroxyacetanilide orN-acetyl-meta-aminophenol (AMAP) is generally regarded as a non-hepatotoxic analog of acetaminophen (APAP). Previous studies demonstrated the absence of toxicity after AMAP in mice, hamsters, primary mouse hepatocytes and several cell lines. In contrast, experiments with liver slices suggested that it may be toxic to human hepatocytes; however, the mechanism of toxicity is unclear. To explore this,we treated primary human hepatocytes (PHH) with AMAP or APAP for up to 48 h and measured several parameters to assess metabolism and injury. Although less toxic than APAP, AMAP dose-dependently triggered cell death in PHH as indicated by alanine aminotransferase (ALT) release and propidium iodide (PI) staining. Similar to APAP, AMAP also significantly depleted glutathione (GSH) in PHH and caused mitochondrial damage as indicated by glutamate dehydrogenase (GDH) release and the JC-1 assay. However, unlike APAP, AMAP treatment did not cause relevant c-jun-N-terminal kinase (JNK) activation in the cytosol or phospho-JNK translocation to mitochondria. To compare, AMAP toxicity was assessed in primary mouse hepatocytes (PMH). No cytotoxicity was observed as indicated by the lack of lactate dehydrogenase release and no PI staining. Furthermore, there was no GSH depletion or mitochondrial dysfunction after AMAP treatment in PMH. Immunoblotting for arylated proteins suggested that AMAP treatment caused extensive mitochondrial protein adduct formation in PHH but not in PMH. In conclusion, AMAP is hepatotoxic in PHH and the mechanism involves the formation of mitochondrial protein adducts and mitochondrial dysfunction.
Keywords: 3′-Hydroxyacetanilide (AMAP); Acetaminophen; Hepatotoxicity; Mitochondrial dysfunction; Protein adducts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Editor's Highlight: Metformin Protects Against Acetaminophen Hepatotoxicity by Attenuation of Mitochondrial Oxidant Stress and Dysfunction.Toxicol Sci. 2016 Dec;154(2):214-226. doi: 10.1093/toxsci/kfw158. Epub 2016 Aug 25. Toxicol Sci. 2016. PMID: 27562556 Free PMC article.
-
Mechanisms of acetaminophen-induced cell death in primary human hepatocytes.Toxicol Appl Pharmacol. 2014 Sep 15;279(3):266-274. doi: 10.1016/j.taap.2014.05.010. Epub 2014 Jun 3. Toxicol Appl Pharmacol. 2014. PMID: 24905542 Free PMC article.
-
Low Dose Acetaminophen Induces Reversible Mitochondrial Dysfunction Associated with Transient c-Jun N-Terminal Kinase Activation in Mouse Liver.Toxicol Sci. 2016 Mar;150(1):204-15. doi: 10.1093/toxsci/kfv319. Epub 2015 Dec 31. Toxicol Sci. 2016. PMID: 26721299 Free PMC article.
-
Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepatotoxicity.Expert Opin Drug Metab Toxicol. 2015;11(11):1769-79. doi: 10.1517/17425255.2015.1071353. Epub 2015 Jul 20. Expert Opin Drug Metab Toxicol. 2015. PMID: 26190663 Free PMC article. Review.
-
Acetaminophen hepatotoxicity: A mitochondrial perspective.Adv Pharmacol. 2019;85:195-219. doi: 10.1016/bs.apha.2019.01.007. Epub 2019 Feb 21. Adv Pharmacol. 2019. PMID: 31307587 Free PMC article. Review.
Cited by
-
Role of autophagy in alcohol and drug-induced liver injury.Food Chem Toxicol. 2020 Feb;136:111075. doi: 10.1016/j.fct.2019.111075. Epub 2019 Dec 23. Food Chem Toxicol. 2020. PMID: 31877367 Free PMC article. Review.
-
Isolation, culture, and delivery considerations for the use of mesenchymal stem cells in potential therapies for acute liver failure.Front Immunol. 2023 Sep 7;14:1243220. doi: 10.3389/fimmu.2023.1243220. eCollection 2023. Front Immunol. 2023. PMID: 37744328 Free PMC article. Review.
-
Role and mechanisms of autophagy in acetaminophen-induced liver injury.Liver Int. 2018 Aug;38(8):1363-1374. doi: 10.1111/liv.13866. Epub 2018 May 14. Liver Int. 2018. PMID: 29682868 Free PMC article. Review.
-
Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity.Food Chem Toxicol. 2017 Oct;108(Pt A):339-350. doi: 10.1016/j.fct.2017.08.020. Epub 2017 Aug 18. Food Chem Toxicol. 2017. PMID: 28827156 Free PMC article.
-
The development and hepatotoxicity of acetaminophen: reviewing over a century of progress.Drug Metab Rev. 2020 Nov;52(4):472-500. doi: 10.1080/03602532.2020.1832112. Epub 2020 Oct 14. Drug Metab Rev. 2020. PMID: 33103516 Free PMC article. Review.
References
-
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–17. - PubMed
-
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–25. - PubMed
-
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. - PubMed
-
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

