The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease
- PMID: 26431937
- PMCID: PMC4592743
- DOI: 10.1016/j.it.2015.08.007
The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease
Abstract
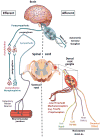
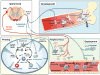
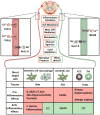
The nervous system and the immune system are the principal sensory interfaces between the internal and external environment. They are responsible for recognizing, integrating, and responding to varied stimuli, and have the capacity to form memories of these encounters leading to learned or 'adaptive' future responses. We review current understanding of the cross-regulation between these systems. The autonomic and somatosensory nervous systems regulate both the development and deployment of immune cells, with broad functions that impact on hematopoiesis as well as on priming, migration, and cytokine production. In turn, specific immune cell subsets contribute to homeostatic neural circuits such as those controlling metabolism, hypertension, and the inflammatory reflex. We examine the contribution of the somatosensory system to autoimmune, autoinflammatory, allergic, and infectious processes in barrier tissues and, in this context, discuss opportunities for therapeutic manipulation of neuro-immune interactions.
Keywords: Neuroscience; barrier tissues; homeostasis; immunology.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
References
-
- Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417–428. - PubMed
Publication types
MeSH terms
Grants and funding
- AI111595/AI/NIAID NIH HHS/United States
- T32 HD055148/HD/NICHD NIH HHS/United States
- AI112521/AI/NIAID NIH HHS/United States
- AI095261/AI/NIAID NIH HHS/United States
- R01 AI111595/AI/NIAID NIH HHS/United States
- R01 AR068383/AR/NIAMS NIH HHS/United States
- P01 AI112521/AI/NIAID NIH HHS/United States
- AI069259/AI/NIAID NIH HHS/United States
- R01 AI069259/AI/NIAID NIH HHS/United States
- U19 AI095261/AI/NIAID NIH HHS/United States
- AR063546/AR/NIAMS NIH HHS/United States
- F31 AR063546/AR/NIAMS NIH HHS/United States
- AR068383/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

