Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability
- PMID: 26431948
- PMCID: PMC6044435
- DOI: 10.1016/j.immuni.2015.09.003
Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability
Erratum in
- Immunity. 2015 Nov 17;43(5):1022. Gulan, Fatih [corrected to Gulen, Muhammet F]
Abstract
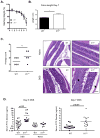
Whether interleukin-17A (IL-17A) has pathogenic and/or protective roles in the gut mucosa is controversial and few studies have analyzed specific cell populations for protective functions within the inflamed colonic tissue. Here we have provided evidence for IL-17A-dependent regulation of the tight junction protein occludin during epithelial injury that limits excessive permeability and maintains barrier integrity. Analysis of epithelial cells showed that in the absence of signaling via the IL-17 receptor adaptor protein Act-1, the protective effect of IL-17A was abrogated and inflammation was enhanced. We have demonstrated that after acute intestinal injury, IL-23R(+) γδ T cells in the colonic lamina propria were the primary producers of early, gut-protective IL-17A, and this production of IL-17A was IL-23 independent, leaving protective IL-17 intact in the absence of IL-23. These results suggest that IL-17-producing γδ T cells are important for the maintenance and protection of epithelial barriers in the intestinal mucosa.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Gut-Busters: IL-17 Ain't Afraid of No IL-23.Immunity. 2015 Oct 20;43(4):620-2. doi: 10.1016/j.immuni.2015.10.001. Immunity. 2015. PMID: 26488809 Free PMC article.
References
-
- Balda MS, Matter K. Tight junctions at a glance. J Cell Sci. 2008;121:3677–3682. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

