Evaluation of small molecule SecA inhibitors against methicillin-resistant Staphylococcus aureus
- PMID: 26432604
- PMCID: PMC4661110
- DOI: 10.1016/j.bmc.2015.09.027
Evaluation of small molecule SecA inhibitors against methicillin-resistant Staphylococcus aureus
Abstract
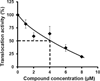
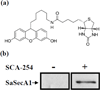
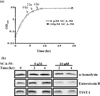
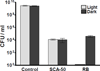
Due to the emergence and rapid spread of drug resistance in bacteria, there is an urgent need for the development of novel antimicrobials. SecA, a key component of the general bacterial secretion system required for viability and virulence, is an attractive antimicrobial target. Earlier we reported that systematical dissection of a SecA inhibitor, Rose Bengal (RB), led to the development of novel small molecule SecA inhibitors active against Escherichia coli and Bacillus subtilis. In this study, two potent RB analogs were further evaluated for activities against methicillin-resistant Staphylococcus aureus (MRSA) strains and for their mechanism of actions. These analogs showed inhibition on the ATPase activities of S. aureus SecA1 (SaSecA1) and SecA2 (SaSecA2), and inhibition of SaSecA1-dependent protein-conducting channel. Moreover, these inhibitors reduce the secretion of three toxins from S. aureus and exert potent bacteriostatic effects against three MRSA strains. Our best inhibitor SCA-50 showed potent concentration-dependent bactericidal activity against MRSA Mu50 strain and very importantly, 2-60 fold more potent inhibitory effect on MRSA Mu50 than all the commonly used antibiotics including vancomycin, which is considered the last resort option in treating MRSA-related infections. Protein pull down experiments further confirmed SaSecA1 as a target. Deletion or overexpression of NorA and MepA efflux pumps had minimal effect on the antimicrobial activities against S. aureus, indicating that the effects of SecA inhibitors were not affected by the presence of these efflux pumps. Our studies show that these small molecule analogs target SecA functions, have potent antimicrobial activities, reduce the secretion of toxins, and have the ability to overcome the effect efflux pumps, which are responsible for multi-drug resistance. Thus, targeting SecA is an attractive antimicrobial strategy against MRSA.
Keywords: Efflux; MRSA; Rose Bengal analogs; SecA inhibitors; SecA-dependent secretion; SecA-liposomes.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures





References
-
- Calfee DP. Curr Opin Infect Dis. 2012;25:385. - PubMed
-
- Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, Ray SM, Harrison LH, Lynfield R, Dumyati G, Townes JM, Schaffner W, Patel PR, Fridkin SK MRSA Investigators of the Emerging Infections Program Active Bacterial Core surveillance. JAMA. 2010;304:641. - PubMed
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. JAMA. 2007;298:1763. - PubMed
-
- Moellering RC., Jr J Antimicrob Chemother. 2012;67:4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases

