Menopausal Status and Abdominal Obesity Are Significant Determinants of Hepatic Lipid Metabolism in Women
- PMID: 26432801
- PMCID: PMC4845132
- DOI: 10.1161/JAHA.115.002258
Menopausal Status and Abdominal Obesity Are Significant Determinants of Hepatic Lipid Metabolism in Women
Abstract
Background: Android fat distribution (abdominal obesity) is associated with insulin resistance, hepatic steatosis, and greater secretion of large very low-density lipoprotein (VLDL) particles in men. Since abdominal obesity is becoming increasingly prevalent in women, we aimed to investigate the relationship between android fat and hepatic lipid metabolism in pre- and postmenopausal women.
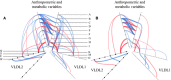
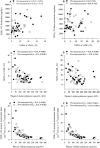
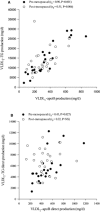
Methods and results: We used a combination of stable isotope tracer techniques to investigate intrahepatic fatty acid synthesis and partitioning in 29 lean and 29 abdominally obese women (android fat/total fat 0.065 [0.02 to 0.08] and 0.095 [0.08 to 0.11], respectively). Thirty women were premenopausal aged 35 to 45 and they were matched for abdominal obesity with 28 postmenopausal women aged 55 to 65. As anticipated, abdominal obese women were more insulin resistant with enhanced hepatic secretion of large (404±30 versus 268±26 mg/kg lean mass, P<0.001) but not small VLDL (160±11 versus 142±13). However, postmenopausal status had a pronounced effect on the characteristics of small VLDL particles, which were considerably triglyceride-enriched (production ratio of VLDL2- triglyceride:apolipoprotein B 30±5.3 versus 19±1.6, P<0.05). In contrast to postmenopausal women, there was a tight control of hepatic fatty acid metabolism and triglyceride production in premenopausal women, whereby oxidation (rs=-0.49, P=0.006), de novo lipogenesis (rs=0.55, P=0.003), and desaturation (rs=0.48, P=0.012) were closely correlated with abdominal obesity-driven large VLDL-triglyceride secretion rate.
Conclusions: In women, abdominal obesity is a major driver of hepatic large VLDL particle secretion, whereas postmenopausal status was characterized by increased small VLDL particle size. These data provide a mechanistic basis for the hyperlipidemia observed in postmenopausal obesity.
Keywords: apolipoproteins; cholesterol; lipids; lipoproteins; menopause; women.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures
Similar articles
-
Nonalcoholic fatty liver disease as the transducer of hepatic oversecretion of very-low-density lipoprotein-apolipoprotein B-100 in obesity.Arterioscler Thromb Vasc Biol. 2010 May;30(5):1043-50. doi: 10.1161/ATVBAHA.109.202275. Epub 2010 Feb 11. Arterioscler Thromb Vasc Biol. 2010. PMID: 20150556 Clinical Trial.
-
Gender differences in VLDL1 and VLDL2 triglyceride kinetics and fatty acid kinetics in obese postmenopausal women and obese men.J Clin Endocrinol Metab. 2012 Jul;97(7):2475-81. doi: 10.1210/jc.2011-3248. Epub 2012 Apr 16. J Clin Endocrinol Metab. 2012. PMID: 22508714
-
Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease.Gastroenterology. 2008 Feb;134(2):424-31. doi: 10.1053/j.gastro.2007.11.038. Epub 2007 Nov 28. Gastroenterology. 2008. PMID: 18242210 Free PMC article.
-
Influence of plasma free fatty acids on lipoprotein synthesis and diabetic dyslipidemia.Exp Clin Endocrinol Diabetes. 2003 Aug;111(5):246-50. doi: 10.1055/s-2003-41284. Exp Clin Endocrinol Diabetes. 2003. PMID: 12951628 Review.
-
Metabolic-associated fatty liver disease and lipoprotein metabolism.Mol Metab. 2021 Aug;50:101238. doi: 10.1016/j.molmet.2021.101238. Epub 2021 Apr 20. Mol Metab. 2021. PMID: 33892169 Free PMC article. Review.
Cited by
-
Case Report: Metreleptin Treatment in a Patient With a Novel Mutation for Familial Partial Lipodystrophy Type 3, Presenting With Uncontrolled Diabetes and Insulin Resistance.Front Endocrinol (Lausanne). 2021 Jun 8;12:684182. doi: 10.3389/fendo.2021.684182. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34168618 Free PMC article.
-
Relationships between bone mineral density, body composition, and isokinetic strength in postmenopausal women.Bone Rep. 2020 Mar 3;12:100255. doi: 10.1016/j.bonr.2020.100255. eCollection 2020 Jun. Bone Rep. 2020. PMID: 32181269 Free PMC article. Review.
-
Autumn Olive (Elaeagnus umbellata Thunb.) Berries Improve Lipid Metabolism and Delay Aging in Middle-Aged Caenorhabditis elegans.Int J Mol Sci. 2024 Mar 18;25(6):3418. doi: 10.3390/ijms25063418. Int J Mol Sci. 2024. PMID: 38542392 Free PMC article.
-
Contribution of Different Phenotypes of Obesity to Metabolic Abnormalities from a Cross-Sectional Study in the Northwest China.Diabetes Metab Syndr Obes. 2021 Jul 7;14:3111-3121. doi: 10.2147/DMSO.S314935. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 34262315 Free PMC article.
-
Fasting hepatic de novo lipogenesis is not reliably assessed using circulating fatty acid markers.Am J Clin Nutr. 2019 Feb 1;109(2):260-268. doi: 10.1093/ajcn/nqy304. Am J Clin Nutr. 2019. PMID: 30721918 Free PMC article.
References
-
- Ng MK. New perspectives on Mars and Venus: unravelling the role of androgens in gender differences in cardiovascular biology and disease. Heart Lung Circ. 2007;16:185–192. - PubMed
-
- Pasquali R, Vicennati V, Bertazzo D, Casimirri F, Pascal G, Tortelli O, Labate AM. Determinants of sex hormone‐binding globulin blood concentrations in premenopausal and postmenopausal women with different estrogen status. Virgilio‐Menopause‐Health Group. Metabolism. 1997;46:5–9. - PubMed
-
- Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. - PubMed
-
- Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki‐Jarvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3490–3497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

