Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury
- PMID: 26432864
- PMCID: PMC4669340
- DOI: 10.1152/ajplung.00031.2015
Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury
Abstract
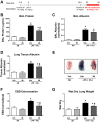
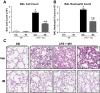
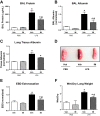
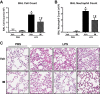
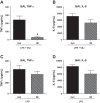
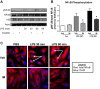
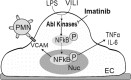
Acute lung injury/acute respiratory distress syndrome (ALI/ARDS), an illness characterized by life-threatening vascular leak, is a significant cause of morbidity and mortality in critically ill patients. Recent preclinical studies and clinical observations have suggested a potential role for the chemotherapeutic agent imatinib in restoring vascular integrity. Our prior work demonstrates differential effects of imatinib in mouse models of ALI, namely attenuation of LPS-induced lung injury but exacerbation of ventilator-induced lung injury (VILI). Because of the critical role of mechanical ventilation in the care of patients with ARDS, in the present study we pursued an assessment of the effectiveness of imatinib in a "two-hit" model of ALI caused by combined LPS and VILI. Imatinib significantly decreased bronchoalveolar lavage protein, total cells, neutrophils, and TNF-α levels in mice exposed to LPS plus VILI, indicating that it attenuates ALI in this clinically relevant model. In subsequent experiments focusing on its protective role in LPS-induced lung injury, imatinib attenuated ALI when given 4 h after LPS, suggesting potential therapeutic effectiveness when given after the onset of injury. Mechanistic studies in mouse lung tissue and human lung endothelial cells revealed that imatinib inhibits LPS-induced NF-κB expression and activation. Overall, these results further characterize the therapeutic potential of imatinib against inflammatory vascular leak.
Keywords: NF-κB; acute lung injury; acute respiratory distress syndrome; endothelium; imatinib; lipopolysaccharide; mechanical ventilation.
Copyright © 2015 the American Physiological Society.
Figures

Similar articles
-
Differential and opposing effects of imatinib on LPS- and ventilator-induced lung injury.Am J Physiol Lung Cell Mol Physiol. 2015 Feb 1;308(3):L259-69. doi: 10.1152/ajplung.00323.2014. Epub 2014 Dec 5. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25480336 Free PMC article.
-
Effect of imatinib on lipopolysaccharide‑induced acute lung injury and endothelial dysfunction through the P38 MAPK and NF-κB signaling pathways in vivo and in vitro.Respir Physiol Neurobiol. 2025 Apr;333:104388. doi: 10.1016/j.resp.2024.104388. Epub 2024 Dec 25. Respir Physiol Neurobiol. 2025. PMID: 39725368
-
Ropivacaine attenuates endotoxin plus hyperinflation-mediated acute lung injury via inhibition of early-onset Src-dependent signaling.BMC Anesthesiol. 2014 Jul 19;14:57. doi: 10.1186/1471-2253-14-57. eCollection 2014. BMC Anesthesiol. 2014. PMID: 25097454 Free PMC article.
-
A long-lasting porcine model of ARDS caused by pneumonia and ventilator-induced lung injury.Crit Care. 2023 Jun 16;27(1):239. doi: 10.1186/s13054-023-04512-8. Crit Care. 2023. PMID: 37328874 Free PMC article. Review.
-
Renin-angiotensin-system, a potential pharmacological candidate, in acute respiratory distress syndrome during mechanical ventilation.Pulm Pharmacol Ther. 2019 Oct;58:101833. doi: 10.1016/j.pupt.2019.101833. Epub 2019 Aug 1. Pulm Pharmacol Ther. 2019. PMID: 31376462 Free PMC article. Review.
Cited by
-
BCR-ABL Tyrosine Kinase Inhibitors: Which Mechanism(s) May Explain the Risk of Thrombosis?TH Open. 2018 Feb 14;2(1):e68-e88. doi: 10.1055/s-0038-1624566. eCollection 2018 Jan. TH Open. 2018. PMID: 31249931 Free PMC article. Review.
-
Clinical Outcomes of Patients with Chronic Myeloid Leukemia and COVID-19 Infection-A Single Center Survey.Medicina (Kaunas). 2023 Aug 28;59(9):1564. doi: 10.3390/medicina59091564. Medicina (Kaunas). 2023. PMID: 37763683 Free PMC article.
-
Emerging pharmacological therapies for ARDS: COVID-19 and beyond.Intensive Care Med. 2020 Dec;46(12):2265-2283. doi: 10.1007/s00134-020-06141-z. Epub 2020 Jul 11. Intensive Care Med. 2020. PMID: 32654006 Free PMC article.
-
Endothelial CLEC5A drives barrier dysfunction and vascular leakage responsible for lung injury in bacterial pneumonia and sepsis.Sci Adv. 2025 Jun 13;11(24):eadt7589. doi: 10.1126/sciadv.adt7589. Epub 2025 Jun 11. Sci Adv. 2025. PMID: 40498836 Free PMC article.
-
Outcome of COVID-19 in patients with chronic myeloid leukemia receiving tyrosine kinase inhibitors.J Oncol Pharm Pract. 2020 Oct;26(7):1676-1682. doi: 10.1177/1078155220953198. Epub 2020 Aug 27. J Oncol Pharm Pract. 2020. PMID: 32854573 Free PMC article.
References
-
- Ventilation with lower tidal volumes compared with traditional tidal volumes for acute lung injury, and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342: 1301–1308, 2000. - PubMed
-
- Akashi N, Matsumoto I, Tanaka Y, Inoue A, Yamamoto K, Umeda N, Hayashi T, Goto D, Ito S, Sekiguchi K, Sumida T. Comparative suppressive effects of tyrosine kinase inhibitors imatinib and nilotinib in models of autoimmune arthritis. Mod Rheumatol 21: 267–275, 2011. - PubMed
-
- Aman J, Peters MJ, Weenink C, van Nieuw Amerongen GP, Vonk Noordegraaf A. Reversal of vascular leak with imatinib. Am J Respir Crit Care Med 188: 1171–1173, 2013. - PubMed
-
- Aman J, van Bezu J, Damanafshan A, Huveneers S, Eringa EC, Vogel SM, Groeneveld AB, Vonk Noordegraaf A, van Hinsbergh VW, van Nieuw Amerongen GP. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation 126: 2728–2738, 2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

