Ecto-5'-nucleotidase CD73 modulates the innate immune response to influenza infection but is not required for development of influenza-induced acute lung injury
- PMID: 26432867
- PMCID: PMC4669338
- DOI: 10.1152/ajplung.00130.2015
Ecto-5'-nucleotidase CD73 modulates the innate immune response to influenza infection but is not required for development of influenza-induced acute lung injury
Abstract
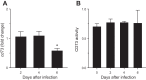
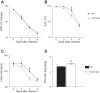
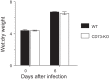
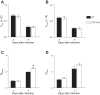
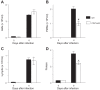
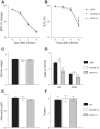
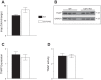
Extracellular nucleotides and nucleosides are important signaling molecules in the lung. Nucleotide and nucleoside concentrations in alveolar lining fluid are controlled by a complex network of surface ectonucleotidases. Previously, we demonstrated that influenza A/WSN/33 (H1N1) virus resulted in increased levels of the nucleotide ATP and the nucleoside adenosine in bronchoalveolar lavage fluid (BALF) of wild-type (WT) C57BL/6 mice. Influenza-induced acute lung injury (ALI) was highly attenuated in A1-adenosine receptor-knockout mice. Because AMP hydrolysis by the ecto-5'-nucleotidase (CD73) plays a central role in and is rate-limiting for generation of adenosine in the normal lung, we hypothesized that ALI would be attenuated in C57BL/6-congenic CD73-knockout (CD73-KO) mice. Infection-induced hypoxemia, bradycardia, viral replication, and bronchoconstriction were moderately increased in CD73-KO mice relative to WT controls. However, postinfection weight loss, pulmonary edema, and parenchymal dysfunction were not altered. Treatment of WT mice with the CD73 inhibitor 5'-(α,β-methylene) diphosphate (APCP) also had no effect on infection-induced pulmonary edema but modestly attenuated hypoxemia. BALF from CD73-KO and APCP-treated WT mice contained more IL-6 and CXCL-10/IFN-γ-induced protein 10, less CXCL-1/keratinocyte chemoattractant, and fewer neutrophils than BALF from untreated WT controls. BALF from APCP-treated WT mice also contained fewer alveolar macrophages and more transforming growth factor-β than BALF from untreated WT mice. These results indicate that CD73 is not necessary for development of ALI following influenza A virus infection and suggest that tissue-nonspecific alkaline phosphatase may be responsible for increased adenosine generation in the infected lung. However, they do suggest that CD73 has a previously unrecognized immunomodulatory role in influenza.
Keywords: CD73; acute lung injury; adenosine; influenza; mouse.
Copyright © 2015 the American Physiological Society.
Figures
References
-
- Bouma M, van der Wildenburg F, Buurman W. Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am J Physiol Cell Physiol 271: C522–C529, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

