Pharmacological targeting of VEGFR signaling with axitinib inhibits Tsc2-null lesion growth in the mouse model of lymphangioleiomyomatosis
- PMID: 26432869
- PMCID: PMC4683311
- DOI: 10.1152/ajplung.00262.2015
Pharmacological targeting of VEGFR signaling with axitinib inhibits Tsc2-null lesion growth in the mouse model of lymphangioleiomyomatosis
Abstract
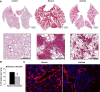
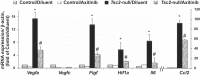
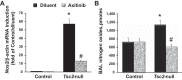
Pulmonary lymphangioleiomyomatosis (LAM), a rare progressive lung disease associated with mutations of the tuberous sclerosis complex 2 (Tsc2) tumor suppressor gene, manifests by neoplastic growth of LAM cells, induction of cystic lung destruction, and respiratory failure. LAM severity correlates with upregulation in serum of the prolymphangiogenic vascular endothelial growth factor D (VEGF-D) that distinguishes LAM from other cystic diseases. The goals of our study was to determine whether Tsc2 deficiency upregulates VEGF-D, and whether axitinib, the Food and Drug Administration-approved small-molecule inhibitor of VEGF receptor (VEGFR) signaling, will reduce Tsc2-null lung lesion growth in a mouse model of LAM. Our data demonstrate upregulation of VEGF-D in the serum and lung lining in mice with Tsc2-null lesions. Progressive growth of Tsc2-null lesions induces recruitment and activation of inflammatory cells and increased nitric oxide production. Recruited cells isolated from the lung lining of mice with Tsc2-null lesions demonstrate upregulated expression of provasculogenic Vegfa, prolymphangiogenic Figf, and proinflammatory Nos2, Il6, and Ccl2 genes. Importantly, axitinib is an effective inhibitor of Tsc2-null lesion growth and inflammatory cell recruitment, which correlates with reduced VEGF-D levels in serum and lung lining. Our data demonstrate that pharmacological inhibition of VEGFR signaling with axitinib inhibits Tsc2-null lesion growth, attenuates recruitment and activation of inflammatory cells, and reduces VEGF-D levels systemically and in the lung lining. Our study suggests a potential therapeutic benefit of inhibition of VEGFR signaling for treatment of LAM.
Keywords: TCS2-null; VEGF-D; animal models; axitinib; lymphangiogenesis.
Copyright © 2015 the American Physiological Society.
Figures



References
-
- Atochina-Vasserman EN, Bates SR, Zhang P, Abramova H, Zhang Z, Gonzales L, Tao JQ, Gochuico BR, Gahl W, Guo CJ, Gow AJ, Beers MF, Guttentag S. Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome. Am J Respir Crit Care Med 184: 449–458, 2011. - PMC - PubMed
-
- Baldwin ME, Roufail S, Halford MM, Alitalo K, Stacker SA, Achen MG. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J Biol Chem 276: 44307–44314, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

