Role of epithelial sodium channels in the regulation of lung fluid homeostasis
- PMID: 26432872
- PMCID: PMC4669342
- DOI: 10.1152/ajplung.00319.2015
Role of epithelial sodium channels in the regulation of lung fluid homeostasis
Abstract
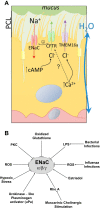
In utero, fetal lung epithelial cells actively secrete Cl(-) ions into the lung air spaces while Na(+) ions follow passively to maintain electroneutrality. This process, driven by an electrochemical gradient generated by the Na(+)-K(+)-ATPase, is responsible for the secretion of fetal fluid that is essential for normal lung development. Shortly before birth, a significant upregulation of amiloride-sensitive epithelial channels (ENaCs) on the apical side of the lung epithelial cells results in upregulation of active Na(+) transport. This process is critical for the reabsorption of fetal lung fluid and the establishment of optimum gas exchange. In the adult lung, active Na(+) reabsorption across distal lung epithelial cells limits the degree of alveolar edema in patients with acute lung injury and cardiogenic edema. Cl(-) ions are transported either paracellularly or transcellularly to preserve electroneutrality. An increase in Cl(-) secretion across the distal lung epithelium has been reported following an acute increase in left atrial pressure and may result in pulmonary edema. In contrast, airway epithelial cells secrete Cl(-) through apical cystic fibrosis transmembrane conductance regulator and Ca(2+)-activated Cl(-) channels and absorb Na(+). Thus the coordinated action of Cl(-) secretion and Na(+) absorption is essential for maintenance of the volume of epithelial lining fluid that, in turn, maximizes mucociliary clearance and facilitates clearance of bacteria and debris from the lungs. Any factor that interferes with Na(+) or Cl(-) transport or dramatically upregulates ENaC activity in airway epithelial cells has been associated with lung diseases such as cystic fibrosis or chronic obstructive lung disease. In this review we focus on the role of the ENaC, the mechanisms involved in ENaC regulation, and how ENaC dysregulation can lead to lung pathology.
Keywords: oxidative stress; plasminogen activator; stem cells; steroid hormones; β-adrenergic agonists.
Copyright © 2015 the American Physiological Society.
Figures
Similar articles
-
Chloride transport-driven alveolar fluid secretion is a major contributor to cardiogenic lung edema.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):E2308-16. doi: 10.1073/pnas.1216382110. Epub 2013 May 3. Proc Natl Acad Sci U S A. 2013. PMID: 23645634 Free PMC article.
-
Salt and water transport across the alveolar epithelium in the developing lung: correlations between function and recent molecular biology advances (Review).Int J Mol Med. 1998 Nov;2(5):515-31. doi: 10.3892/ijmm.2.5.515. Int J Mol Med. 1998. PMID: 9858647 Review.
-
Pulmonary oedema fluid induces non-alpha-ENaC-dependent Na(+) transport and fluid absorption in the distal lung.J Physiol. 2002 Oct 15;544(2):537-48. doi: 10.1113/jphysiol.2002.024612. J Physiol. 2002. PMID: 12381825 Free PMC article.
-
beta-Liddle mutation of the epithelial sodium channel increases alveolar fluid clearance and reduces the severity of hydrostatic pulmonary oedema in mice.J Physiol. 2007 Jul 15;582(Pt 2):777-88. doi: 10.1113/jphysiol.2007.131078. Epub 2007 Apr 12. J Physiol. 2007. PMID: 17430990 Free PMC article.
-
Influenza virus infection alters ion channel function of airway and alveolar cells: mechanisms and physiological sequelae.Am J Physiol Lung Cell Mol Physiol. 2017 Nov 1;313(5):L845-L858. doi: 10.1152/ajplung.00244.2017. Epub 2017 Aug 3. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28775098 Free PMC article. Review.
Cited by
-
Mechanical Strain-Mediated Tenogenic Differentiation of Mesenchymal Stromal Cells Is Regulated through Epithelial Sodium Channels.Stem Cells Int. 2020 Aug 18;2020:5385960. doi: 10.1155/2020/5385960. eCollection 2020. Stem Cells Int. 2020. PMID: 32908542 Free PMC article.
-
The Role of MicroRNA in the Airway Surface Liquid Homeostasis.Int J Mol Sci. 2020 May 28;21(11):3848. doi: 10.3390/ijms21113848. Int J Mol Sci. 2020. PMID: 32481719 Free PMC article. Review.
-
Lipopolysaccharide Inhibits Alpha Epithelial Sodium Channel Expression via MiR-124-5p in Alveolar Type 2 Epithelial Cells.Biomed Res Int. 2020 Mar 3;2020:8150780. doi: 10.1155/2020/8150780. eCollection 2020. Biomed Res Int. 2020. PMID: 32190682 Free PMC article.
-
Respiratory Barrier as a Safeguard and Regulator of Defense Against Influenza A Virus and Streptococcus pneumoniae.Front Immunol. 2020 Feb 4;11:3. doi: 10.3389/fimmu.2020.00003. eCollection 2020. Front Immunol. 2020. PMID: 32117216 Free PMC article. Review.
-
Ion Channel Regulation by Sex Steroid Hormones and Vitamin D in Cancer: A Potential Opportunity for Cancer Diagnosis and Therapy.Front Pharmacol. 2020 Feb 28;11:152. doi: 10.3389/fphar.2020.00152. eCollection 2020. Front Pharmacol. 2020. PMID: 32210800 Free PMC article. Review.
References
-
- Bangel-Ruland N, Sobczak K, Christmann T, Kentrup D, Langhorst H, Kusche-Vihrog K, Weber WM. Characterization of the epithelial sodium channel δ-subunit in human nasal epithelium. Am J Respir Cell Mol Biol 42: 498–505, 2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

