The zebrafish Kupffer's vesicle as a model system for the molecular mechanisms by which the lack of Polycystin-2 leads to stimulation of CFTR
- PMID: 26432887
- PMCID: PMC4728361
- DOI: 10.1242/bio.014076
The zebrafish Kupffer's vesicle as a model system for the molecular mechanisms by which the lack of Polycystin-2 leads to stimulation of CFTR
Abstract
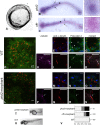
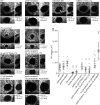
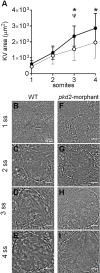
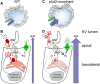
In autosomal dominant polycystic kidney disease (ADPKD), cyst inflation and continuous enlargement are associated with marked transepithelial ion and fluid secretion into the cyst lumen via cystic fibrosis transmembrane conductance regulator (CFTR). Indeed, the inhibition or degradation of CFTR prevents the fluid accumulation within cysts. The in vivo mechanisms by which the lack of Polycystin-2 leads to CFTR stimulation are an outstanding challenge in ADPKD research and may bring important biomarkers for the disease. However, hampering their study, the available ADPKD in vitro cellular models lack the three-dimensional architecture of renal cysts and the ADPKD mouse models offer limited access for live-imaging experiments in embryonic kidneys. Here, we tested the zebrafish Kupffer's vesicle (KV) as an alternative model-organ. KV is a fluid-filled vesicular organ, lined by epithelial cells that express both CFTR and Polycystin-2 endogenously, being each of them easily knocked-down. Our data on the intracellular distribution of Polycystin-2 support its involvement in the KV fluid-flow induced Ca(2+)-signalling. Mirroring kidney cysts, the KV lumen inflation is dependent on CFTR activity and, as we clearly show, the knockdown of Polycystin-2 results in larger KV lumens through overstimulation of CFTR. In conclusion, we propose the zebrafish KV as a model organ to study the renal cyst inflation. Favouring its use, KV volume can be easily determined by in vivo imaging offering a live readout for screening compounds and genes that may prevent cyst enlargement through CFTR inhibition.
Keywords: (ADPKD); (CFTR); (KV); Autosomal dominant polycystic kidney disease; Cystic fibrosis transmembrane conductance regulator; Kupffer's vesicle; Polycystin-2; Zebrafish.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

Similar articles
-
Zebrafish Model as a Screen to Prevent Cyst Inflation in Autosomal Dominant Polycystic Kidney Disease.Int J Mol Sci. 2021 Aug 20;22(16):9013. doi: 10.3390/ijms22169013. Int J Mol Sci. 2021. PMID: 34445719 Free PMC article.
-
Cftr controls lumen expansion and function of Kupffer's vesicle in zebrafish.Development. 2013 Apr;140(8):1703-12. doi: 10.1242/dev.091819. Epub 2013 Mar 13. Development. 2013. PMID: 23487313 Free PMC article.
-
Ouabain Regulates CFTR-Mediated Anion Secretion and Na,K-ATPase Transport in ADPKD Cells.J Membr Biol. 2015 Dec;248(6):1145-57. doi: 10.1007/s00232-015-9832-7. Epub 2015 Aug 20. J Membr Biol. 2015. PMID: 26289599 Free PMC article.
-
[Inhibitors of intra-cystic secretion: novel therapies in ADPKD (Autosomal Dominant Polycystic Kidney Disease)].G Ital Nefrol. 2013 Jan-Feb;30(1):gin/30.1.2. G Ital Nefrol. 2013. PMID: 23832438 Review. Italian.
-
Therapeutic potential of cystic fibrosis transmembrane conductance regulator (CFTR) inhibitors in polycystic kidney disease.BioDrugs. 2009;23(4):203-16. doi: 10.2165/11313570-000000000-00000. BioDrugs. 2009. PMID: 19697963 Review.
Cited by
-
Zebrafish: A Promising Real-Time Model System for Nanotechnology-Mediated Neurospecific Drug Delivery.Nanoscale Res Lett. 2021 Aug 23;16(1):135. doi: 10.1186/s11671-021-03592-1. Nanoscale Res Lett. 2021. PMID: 34424426 Free PMC article. Review.
-
What Role Does CFTR Play in Development, Differentiation, Regeneration and Cancer?Int J Mol Sci. 2020 Apr 29;21(9):3133. doi: 10.3390/ijms21093133. Int J Mol Sci. 2020. PMID: 32365523 Free PMC article. Review.
-
CFTR is required for the migration of primordial germ cells during zebrafish early embryogenesis.Reproduction. 2018 Sep;156(3):261-268. doi: 10.1530/REP-17-0681. Epub 2018 Jun 21. Reproduction. 2018. PMID: 29930176 Free PMC article.
-
Claudin5a is required for proper inflation of Kupffer's vesicle lumen and organ laterality.PLoS One. 2017 Aug 3;12(8):e0182047. doi: 10.1371/journal.pone.0182047. eCollection 2017. PLoS One. 2017. PMID: 28771527 Free PMC article.
-
Tissue Flow Induces Cell Shape Changes During Organogenesis.Biophys J. 2018 Dec 4;115(11):2259-2270. doi: 10.1016/j.bpj.2018.10.028. Epub 2018 Nov 6. Biophys J. 2018. PMID: 30455043 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

