Preferential Destruction of Interstitial Macrophages over Alveolar Macrophages as a Cause of Pulmonary Disease in Simian Immunodeficiency Virus-Infected Rhesus Macaques
- PMID: 26432896
- PMCID: PMC4637238
- DOI: 10.4049/jimmunol.1501194
Preferential Destruction of Interstitial Macrophages over Alveolar Macrophages as a Cause of Pulmonary Disease in Simian Immunodeficiency Virus-Infected Rhesus Macaques
Abstract
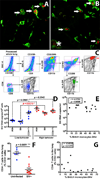
To our knowledge, this study demonstrates for the first time that the AIDS virus differentially impacts two distinct subsets of lung macrophages. The predominant macrophages harvested by bronchoalveolar lavage (BAL), alveolar macrophages (AMs), are routinely used in studies on human lung macrophages, are long-lived cells, and exhibit low turnover. Interstitial macrophages (IMs) inhabit the lung tissue, are not recovered with BAL, are shorter-lived, and exhibit higher baseline turnover rates distinct from AMs. We examined the effects of SIV infection on AMs in BAL fluid and IMs in lung tissue of rhesus macaques. SIV infection produced massive cell death of IMs that contributed to lung tissue damage. Conversely, SIV infection induced minimal cell death of AMs, and these cells maintained the lower turnover rate throughout the duration of infection. This indicates that SIV produces lung tissue damage through destruction of IMs, whereas the longer-lived AMs may serve as a virus reservoir to facilitate HIV persistence.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures



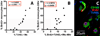
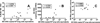
References
-
- Van Rompay KK. The use of nonhuman primate models of HIV infection for the evaluation of antiviral strategies. AIDS research and human retroviruses. 2012;28:16–35. - PubMed
-
- Cai Y, Sugimoto C, Liu DX, Midkiff CC, Alvarez X, Lackner AA, Kim WK, Didier ES, Kuroda MJ. Increased monocyte turnover is associated with interstitial macrophage accumulation and pulmonary tissue damage in SIV-infected rhesus macaques. Journal of leukocyte biology. 2015;97:1147–1153. - PMC - PubMed
-
- Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 AI110163/AI/NIAID NIH HHS/United States
- R21 AI087302/AI/NIAID NIH HHS/United States
- P51OD011104/OD/NIH HHS/United States
- HL125054/HL/NHLBI NIH HHS/United States
- P51 RR000164/RR/NCRR NIH HHS/United States
- R01 AI097059/AI/NIAID NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
- R21 AI116198/AI/NIAID NIH HHS/United States
- AI116198/AI/NIAID NIH HHS/United States
- R01 HL125054/HL/NHLBI NIH HHS/United States
- AI110163/AI/NIAID NIH HHS/United States
- AI091501/AI/NIAID NIH HHS/United States
- AI097059/AI/NIAID NIH HHS/United States
- AI087302/AI/NIAID NIH HHS/United States
- R33 AI110163/AI/NIAID NIH HHS/United States
- R21 AI091501/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

