Endocrine and other physiologic modulators of perinatal cardiomyocyte endowment
- PMID: 26432905
- PMCID: PMC4677998
- DOI: 10.1530/JOE-15-0309
Endocrine and other physiologic modulators of perinatal cardiomyocyte endowment
Abstract
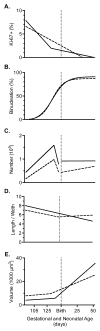
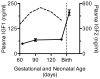
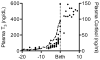
Immature contractile cardiomyocytes proliferate to rapidly increase cell number, establishing cardiomyocyte endowment in the perinatal period. Developmental changes in cellular maturation, size and attrition further contribute to cardiac anatomy. These physiological processes occur concomitant with a changing hormonal environment as the fetus prepares itself for the transition to extrauterine life. There are complex interactions between endocrine, hemodynamic and nutritional regulators of cardiac development. Birth has been long assumed to be the trigger for major differences between the fetal and postnatal cardiomyocyte growth patterns, but investigations in normally growing sheep and rodents suggest this may not be entirely true; in sheep, these differences are initiated before birth, while in rodents they occur after birth. The aim of this review is to draw together our understanding of the temporal regulation of these signals and cardiomyocyte responses relative to birth. Further, we consider how these dynamics are altered in stressed and suboptimal intrauterine environments.
Keywords: angiotensin II; birth; cardiomyocyte growth; cortisol; heart development; hypertension; hypoxia; insulin-like growth factor 1; terminal differentiation; thyroid hormone.
© 2016 Society for Endocrinology.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Figures
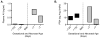
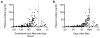
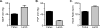
References
-
- Adler CP. Relationship between deoxyribonucleic acid content and nucleoli in human heart muscle cells and estimation of cell number during cardiac growth and hyperfunction. Recent Advances In Studies On Cardiac Structure and Metabolism. 1975;8:373–386. - PubMed
-
- Adler CP, Costabel U. Cell number in human heart in atrophy, hypertrophy, and under the influence of cytostatics. Recent Adv Stud Cardiac Struct Metab. 1975;6:343–355. - PubMed
-
- Adler CP, Costabel U. Myocardial DNA and cell number under the influence of cytostatics. I. Post mortem investigations of human hearts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;32:109–125. - PubMed
-
- Adler CP, Friedburg H, Herget GW, Neuburger M, Schwalb H. Variability of cardiomyocyte DNA content, ploidy level and nuclear number in mammalian hearts. Virchows Archiv. 1996;429:159–164. - PubMed
-
- Anderson DF, Barbera A, Faber JJ. Substantial reductions in blood pressure after bilateral nephrectomy in fetal sheep. Am J Physiol. 1994;266:H17–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

