A quantitative framework for whole-body coordination reveals specific deficits in freely walking ataxic mice
- PMID: 26433022
- PMCID: PMC4630674
- DOI: 10.7554/eLife.07892
A quantitative framework for whole-body coordination reveals specific deficits in freely walking ataxic mice
Abstract
The coordination of movement across the body is a fundamental, yet poorly understood aspect of motor control. Mutant mice with cerebellar circuit defects exhibit characteristic impairments in locomotor coordination; however, the fundamental features of this gait ataxia have not been effectively isolated. Here we describe a novel system (LocoMouse) for analyzing limb, head, and tail kinematics of freely walking mice. Analysis of visibly ataxic Purkinje cell degeneration (pcd) mice reveals that while differences in the forward motion of individual paws are fully accounted for by changes in walking speed and body size, more complex 3D trajectories and, especially, inter-limb and whole-body coordination are specifically impaired. Moreover, the coordination deficits in pcd are consistent with a failure to predict and compensate for the consequences of movement across the body. These results isolate specific impairments in whole-body coordination in mice and provide a quantitative framework for understanding cerebellar contributions to coordinated locomotion.
Keywords: Purkinje cell; ataxia; cerebellum; locomotion; mouse; neuroscience.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


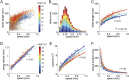
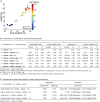

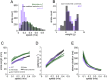
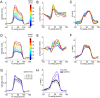




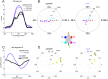
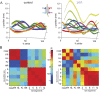


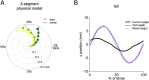
References
-
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. Journal of Neurophysiology. 1996;76:492–509. - PubMed
-
- Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0-5. 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

