Activation of signal transduction pathways during hepatic oncogenesis
- PMID: 26433160
- PMCID: PMC4684747
- DOI: 10.1016/j.canlet.2015.09.016
Activation of signal transduction pathways during hepatic oncogenesis
Abstract
Background and aims: Understanding the molecular pathogenesis of hepatocellular carcinoma (HCC) is essential to identify therapeutic targets. A hepatitis B virus (HBV) related double transgenic murine model was developed.
Methods: Liver specific expression of HBV X protein (HBx) and insulin receptor substrate 1 (IRS1) was achieved and transgenic mice were followed from birth to age 21 months. Liver and tumor tissue were assessed for histologic changes as well as activation of signal transduction pathways by qRT-PCR and multiplex ELISA protein assays.
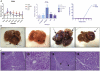
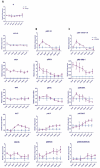
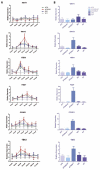
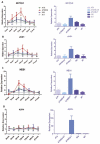
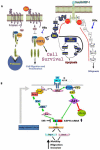
Results: Overexpression of HBx and IRS1 stimulates liver cell proliferation in the double transgenic mice. Only the male mice developed HCC starting at age 15-18 months. The IN/IGF1/IRS1/MAPK/ERK and IN/IGF1/IRS1/PI3K/AKT/GSK3β cascades were activated early (6-9 months) in the liver followed by WNT/β-catenin and Notch signaling. Aspartate β-hydroxylase (ASPH) was found to link these upstream growth factor signaling pathways to downstream Notch activation in tumor tissues.
Conclusions: Sustained overexpression of HBx and IRS1 led to constitutive activation of a tripartite growth factor signal transduction cascade in the liver and was necessary and sufficient to promote HCC development and progression.
Keywords: Growth factor signaling pathways; Hepatocellular carcinoma; Transgenic mice.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures
References
-
- Hollinger FB, Purcell RH, Gerin JL, Ganem DE, Feinstone SM. Viral Hepatitis. 1st Edition Lippincott Williams & Wilkins; 2002.
-
- Schluter V, Meyer M, Hofschneider PH, Koshy R, Caselmann WH. Integrated hepatitis B virus X and 3′ truncated preS/S sequences derived from human hepatomas encode functionally active transactivators. Oncogene. 1994;9:3335–3344. - PubMed
-
- Nishiyama M, Wands JR. Cloning and increased expression of an insulin receptor substrate-1-like gene in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1992;183:280–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

