Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC)
- PMID: 26433246
- PMCID: PMC5026117
- DOI: 10.1016/j.jaad.2015.08.041
Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC)
Abstract
Background: Merkel cell carcinoma (MCC) is a neuroendocrine carcinoma, associated with Merkel cell polyomavirus. MCC admixed with squamous cell carcinoma (SCC) is unassociated with polyomavirus, and is genetically distinct.
Objective: We sought to distinguish clinically and dermoscopically between MCC and SCC/MCC.
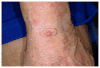
Methods: We compared patient data for SCC/MCC (n = 26) and MCC (n = 20), and reviewed clinical and dermoscopic images (n = 9) of SCC/MCC.
Results: Patients with SCC/MCC were older (median 76.5 vs 69 years) and more often male (77% vs 60%), and had more nonmelanoma skin cancer (85% vs 25%), malignant extracutaneous tumors (25% vs 5%), lymphoproliferative disorders (23% vs 10%), and immunodeficient/proinflammatory states (77% vs 35%). In all, 58% of SCC/MCC versus 10% of MCC were clinically diagnosed nonmelanoma skin cancer. Patients with SCC/MCC had more metastases (77% vs 40%), more treatment failures (53% vs 45%), shorter survival (41 vs 54 months), and more death from disease (50% vs 40%). SCC/MCC demonstrated marked scale (7/9), and telangiectasia (1/9). Dermoscopically, small dotted and short linear irregular peripheral vessels and central milky-red areas with large-diameter arborizing vessels were seen.
Limitations: The rarity of SCC/MCC limits available data.
Conclusions: SCC/MCC is aggressive, arising within elderly patients' chronically ultraviolet-exposed skin, often in the setting of immunosuppression or inflammation. Dermoscopically, polymorphous vessels in lesions suspicious for nonmelanoma skin cancer are suggestive.
Keywords: Merkel cell; biphenotypic; dermoscopy; neuroendocrine carcinoma; polyomavirus; ultraviolet signature.
Copyright © 2015 American Academy of Dermatology, Inc. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Bichakjian CK, Lowe L, Lao CD, et al. Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer. 2007;110(1):1–12. - PubMed
-
- Martin B, Poblet E, Rios JJ, et al. Merkel cell carcinoma with divergent differentiation: histopathological and immunohistochemical study of 15 cases with PCR analysis for Merkel cell polyomavirus. Histopathology. 2013;62(5):711–722. - PubMed
-
- Paik JY, Hall G, Clarkson A, et al. Immunohistochemistry for Merkel cell polyomavirus is highly specific but not sensitive for the diagnosis of Merkel cell carcinoma in the Australian population. Hum Pathol. 2011;42(10):1385–1390. - PubMed
-
- Kuwamoto S. Recent advances in the biology of Merkel cell carcinoma. Hum Pathol. 2011;42(8):1063–1077. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

