Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents?
- PMID: 26433697
- PMCID: PMC7127033
- DOI: 10.1016/j.tim.2015.07.005
Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents?
Abstract
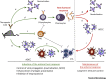
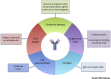
Monoclonal antibodies (mAbs) are increasingly being considered as agents to fight severe viral diseases. So far, they have essentially been selected and used on the basis of their virus-neutralizing activity and/or cell-killing activity to blunt viral propagation via direct mechanisms. There is, however, accumulating evidence that they can also induce long-lasting protective antiviral immunity by recruiting the endogenous immune system of infected individuals during the period of immunotherapy. Exploiting this property may revolutionize antiviral mAb-based immunotherapies, with benefits for both patients and healthcare systems.
Keywords: Fc receptors; antiviral therapy; immune complexes; immunotherapy; monoclonal antibodies; vaccine-like effects.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

References
-
- Beck A. Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 2010;10:345–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

