Susceptibility of Clostridium difficile isolates from a Phase 2 clinical trial of cadazolid and vancomycin in C. difficile infection
- PMID: 26433782
- PMCID: PMC4681371
- DOI: 10.1093/jac/dkv300
Susceptibility of Clostridium difficile isolates from a Phase 2 clinical trial of cadazolid and vancomycin in C. difficile infection
Abstract
Objectives: The aim of this study was to evaluate the susceptibilities of Clostridium difficile isolates to cadazolid, a novel antibiotic for the treatment of C. difficile infection.
Methods: Ribotyping and susceptibilities were determined for C. difficile isolates from a multicentre, double-blind, Phase 2 study of oral cadazolid in patients with C. difficile infection (NCT01222702, ClinicalTrials.gov; EudraCT 2010-020941-29, European Clinical Trials Database). Patients were randomized to receive 250, 500 or 1000 mg of cadazolid twice daily or 125 mg of vancomycin four times daily, for 10 days. MICs of cadazolid, vancomycin, fidaxomicin, linezolid and moxifloxacin were determined at baseline for all patients and post-baseline for patients with clinical failure or recurrence, using the agar dilution method.
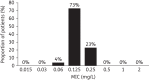
Results: Seventy-eight of 84 patients had an evaluable toxigenic C. difficile isolate at baseline. The most frequent PCR ribotype was 027 (15.4%). Cadazolid MICs for baseline isolates (including epidemic strain 027) ranged from 0.06 to 0.25 mg/L. Baseline cadazolid MICs were similar to those of fidaxomicin and lower than those of vancomycin, linezolid and moxifloxacin. For each clinical outcome group (clinical cure, clinical failure, sustained clinical response and clinical failure or recurrence), the baseline cadazolid MIC range was 0.06-0.25 mg/L. Mean (min-max) cadazolid faecal concentration (μg/g) on day 5 was 884 (101-2710), 1706 (204-4230) and 3226 (1481-12 600) for the doses 250, 500 and 1000 mg, respectively.
Conclusions: For all cadazolid doses, the faecal concentration was in excess of several thousand-fold the MIC90 for C. difficile. The MIC of cadazolid for all C. difficile isolates, including epidemic strains, was low and in the same narrow range regardless of treatment outcome.
© The Author 2015. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures
References
-
- McDonald LC, Killgore GE, Thompson A et al. . An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005; 353: 2433–41. - PubMed
-
- Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 2009; 7: 526–36. - PubMed
-
- Jones AM, Kuijper EJ, Wilcox MH. Clostridium difficile: a European perspective. J Infect 2013; 66: 115–28. - PubMed
-
- Borgmann S, Kist M, Jakobiak T et al. . Increased number of Clostridium difficile infections and prevalence of Clostridium difficile PCR ribotype 001 in southern Germany. Euro Surveill 2008; 13: pii=19057. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical