Impaired mitochondrial network excitability in failing guinea-pig cardiomyocytes
- PMID: 26433944
- PMCID: PMC4692289
- DOI: 10.1093/cvr/cvv230
Impaired mitochondrial network excitability in failing guinea-pig cardiomyocytes
Abstract
Aims: Studies in guinea-pig cardiomyocytes show that reactive oxygen species (ROS) produced by a few mitochondria can propagate to their neighbours, triggering synchronized, cell-wide network oscillations via an ROS-induced ROS release (RIRR) mechanism. How mitochondria in cardiomyocytes from failing hearts (HF) respond to local oxidative stress perturbations has not been investigated. Since mitochondrial ultrastructure is reportedly disrupted in HF, and propagation of ROS signals depends on mitochondrial network integrity, we hypothesized that the laser flash-induced RIRR is altered in HF.
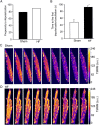
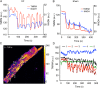
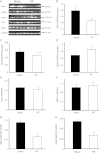
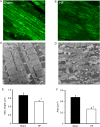
Methods and results: To test the hypothesis, pressure-overload HF was induced in guinea pigs by ascending aortic constriction leading to left ventricular dilatation and decreased ejection fraction after 8 weeks. Isolated cardiomyocytes were studied with two-photon/confocal microscopy to determine their basal oxidative stress and propensity to undergo mitochondrial depolarization/oscillations in response to local laser flash stimulations. The expression of mitofusin proteins and mitochondrial network structure were also analysed. Results showed that HF cardiomyocytes had higher baseline ROS levels and less reduced glutathione, and were more prone to laser flash-induced mitochondrial depolarization. In contrast, the delay between the laser flash and synchronized cell-wide network oscillations was prolonged in HF myocytes compared with shams, and the spatial extent of coupling was diminished, suggesting dampened RIRR and ROS signal propagation. In addition, the expressions of mitofusin proteins in HF myocardium were down-regulated compared with these from sham-operated animals, and the mitochondrial network structure altered.
Conclusion: The disrupted inter-mitochondrial tethering and loss of structural organization may underlie decreased ROS-dependent mitochondrial coupling in HF.
Keywords: Excitability; Heart failure; Mitochondrial network; Mitofusin protein; ROS-induced-ROS release.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2015. For permissions please email: journals.permissions@oup.com.
Figures

Comment in
-
Orphaned mitochondria in heart failure.Cardiovasc Res. 2016 Jan 1;109(1):6-8. doi: 10.1093/cvr/cvv262. Epub 2015 Nov 29. Cardiovasc Res. 2016. PMID: 26621822 No abstract available.
References
-
- Bers DM, Despa S. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J Pharmacol Sci 2006;100:315–322. - PubMed
-
- Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation 1999;100:999–1008. - PubMed
-
- Pieske B, Maier LS, Piacentino V III, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation 2002;106:447–453. - PubMed
-
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 2005;85:1093–1129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

