Atypical role of sprouty in colorectal cancer: sprouty repression inhibits epithelial-mesenchymal transition
- PMID: 26434583
- PMCID: PMC4850112
- DOI: 10.1038/onc.2015.365
Atypical role of sprouty in colorectal cancer: sprouty repression inhibits epithelial-mesenchymal transition
Erratum in
-
Atypical role of sprouty in colorectal cancer: sprouty repression inhibits epithelial-mesenchymal transition.Oncogene. 2017 Jul 13;36(28):4088. doi: 10.1038/onc.2017.56. Epub 2017 Mar 13. Oncogene. 2017. PMID: 28288131 Free PMC article.
Abstract
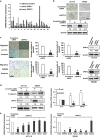
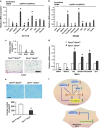
Sprouty (SPRY) appears to act as a tumor suppressor in cancer, whereas we demonstrated that SPRY2 functions as a putative oncogene in colorectal cancer (CRC) (Oncogene, 2010, 29: 5241-5253). We investigated the mechanisms by which SPRY regulates epithelial-mesenchymal transition (EMT) in CRC. SPRY1 and SPRY2 mRNA transcripts were significantly upregulated in human CRC. Suppression of SPRY2 repressed AKT2 and EMT-inducing transcription factors and significantly increased E-cadherin expression. Concurrent downregulation of SPRY1 and SPRY2 also increased E-cadherin and suppressed mesenchymal markers in colon cancer cells. An inverse expression pattern between AKT2 and E-cadherin was established in a human CRC tissue microarray. SPRY2 negatively regulated miR-194-5p that interacts with AKT2 3' untranslated region. Mir-194 mimics increased E-cadherin expression and suppressed cancer cell migration and invasion. By confocal microscopy, we demonstrated redistribution of E-cadherin to plasma membrane in colon cancer cells transfected with miR-194. Spry1(-/-) and Spry2(-/-) double mutant mouse embryonic fibroblasts exhibited decreased cell migration while acquiring several epithelial markers. In CRC, SPRY drive EMT and may serve as a biomarker of poor prognosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Atypical role of sprouty in p21 dependent inhibition of cell proliferation in colorectal cancer.Mol Carcinog. 2016 Sep;55(9):1355-68. doi: 10.1002/mc.22379. Epub 2015 Aug 21. Mol Carcinog. 2016. PMID: 26293890 Free PMC article.
-
Spry1 and Spry2 are necessary for eyelid closure.Dev Biol. 2013 Nov 15;383(2):227-38. doi: 10.1016/j.ydbio.2013.09.014. Epub 2013 Sep 17. Dev Biol. 2013. PMID: 24055172 Free PMC article.
-
CHIP functions as an oncogene by promoting colorectal cancer metastasis via activation of MAPK and AKT signaling and suppression of E-cadherin.J Transl Med. 2018 Jun 19;16(1):169. doi: 10.1186/s12967-018-1540-5. J Transl Med. 2018. PMID: 29921293 Free PMC article.
-
Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer.J Hematol Oncol. 2017 Mar 29;10(1):79. doi: 10.1186/s13045-017-0445-8. J Hematol Oncol. 2017. PMID: 28356122 Free PMC article.
-
SPROUTY-2 represses the epithelial phenotype of colon carcinoma cells via upregulation of ZEB1 mediated by ETS1 and miR-200/miR-150.Oncogene. 2016 Jun 9;35(23):2991-3003. doi: 10.1038/onc.2015.366. Epub 2015 Oct 12. Oncogene. 2016. PMID: 26455323
Cited by
-
Identification of stage-associated exosome miRNAs in colorectal cancer by improved robust and corroborative approach embedded miRNA-target network.Front Med (Lausanne). 2022 Sep 27;9:881788. doi: 10.3389/fmed.2022.881788. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36237545 Free PMC article.
-
Epigenetic DNA Modifications Upregulate SPRY2 in Human Colorectal Cancers.Cells. 2021 Oct 2;10(10):2632. doi: 10.3390/cells10102632. Cells. 2021. PMID: 34685612 Free PMC article.
-
Long non‑coding RNA Unigene56159 promotes glioblastoma multiforme cell proliferation and invasion through negatively regulating microRNA‑194‑5p.Mol Med Rep. 2020 Feb;21(2):768-776. doi: 10.3892/mmr.2019.10852. Epub 2019 Nov 26. Mol Med Rep. 2020. PMID: 31789416 Free PMC article.
-
Loss of Spry1 reduces growth of BRAFV600-mutant cutaneous melanoma and improves response to targeted therapy.Cell Death Dis. 2020 May 22;11(5):392. doi: 10.1038/s41419-020-2585-y. Cell Death Dis. 2020. PMID: 32444628 Free PMC article.
-
Knockdown of SOX2OT inhibits the malignant biological behaviors of glioblastoma stem cells via up-regulating the expression of miR-194-5p and miR-122.Mol Cancer. 2017 Nov 13;16(1):171. doi: 10.1186/s12943-017-0737-1. Mol Cancer. 2017. PMID: 29132362 Free PMC article.
References
-
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell 1998; 92: 253–263. - PubMed
-
- Leeksma OC, Van Achterberg TA, Tsumura Y, Toshima J, Eldering E, Kroes WG et al. Human sprouty 4, a new ras antagonist on 5q31, interacts with the dual specificity kinase TESK1. Eur J Biochem 2002; 269: 2546–2556. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

