Diabetes and ageing-induced vascular inflammation
- PMID: 26435167
- PMCID: PMC4933100
- DOI: 10.1113/JP270841
Diabetes and ageing-induced vascular inflammation
Abstract
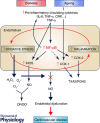
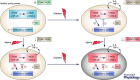
Diabetes and the ageing process independently increase the risk for cardiovascular disease (CVD). Since incidence of diabetes increases as people get older, the diabetic older adults represent the largest population of diabetic subjects. This group of patients would potentially be threatened by the development of CVD related to both ageing and diabetes. The relationship between CVD, ageing and diabetes is explained by the negative impact of these conditions on vascular function. Functional and clinical evidence supports the role of vascular inflammation induced by the ageing process and by diabetes in vascular impairment and CVD. Inflammatory mechanisms in both aged and diabetic vasculature include pro-inflammatory cytokines, vascular hyperactivation of nuclear factor-кB, increased expression of cyclooxygenase and inducible nitric oxide synthase, imbalanced expression of pro/anti-inflammatory microRNAs, and dysfunctional stress-response systems (sirtuins, Nrf2). In contrast, there are scarce data regarding the interaction of these mechanisms when ageing and diabetes co-exist and its impact on vascular function. Older diabetic animals and humans display higher vascular impairment and CVD risk than those either aged or diabetic, suggesting that chronic low-grade inflammation in ageing creates a vascular environment favouring the mechanisms of vascular damage driven by diabetes. Further research is needed to determine the specific inflammatory mechanisms responsible for exacerbated vascular impairment in older diabetic subjects in order to design effective therapeutic interventions to minimize the impact of vascular inflammation. This would help to prevent or delay CVD and the specific clinical manifestations (cognitive decline, frailty and disability) promoted by diabetes-induced vascular impairment in the elderly.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures

References
-
- Ahmad M, Turkseven S, Mingone CJ, Gupte SA, Wolin MS & Abraham NG (2005). Heme oxygenase‐1 gene expression increases vascular relaxation and decreases inducible nitric oxide synthase in diabetic rats. Cell Mol Biol (Noisy‐le‐grand) 51, 371–376. - PubMed
-
- Alvarez de Sotomayor M, Mingorance C & Andriantsitohaina R (2007). Fenofibrate improves age‐related endothelial dysfunction in rat resistance arteries. Atherosclerosis 193, 112–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

