Plasma membrane restricted RhoGEF activity is sufficient for RhoA-mediated actin polymerization
- PMID: 26435194
- PMCID: PMC4592971
- DOI: 10.1038/srep14693
Plasma membrane restricted RhoGEF activity is sufficient for RhoA-mediated actin polymerization
Abstract
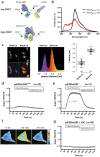
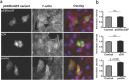
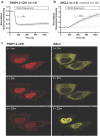
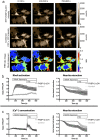
The small GTPase RhoA is involved in cell morphology and migration. RhoA activity is tightly regulated in time and space and depends on guanine exchange factors (GEFs). However, the kinetics and subcellular localization of GEF activity towards RhoA are poorly defined. To study the mechanism underlying the spatiotemporal control of RhoA activity by GEFs, we performed single cell imaging with an improved FRET sensor reporting on the nucleotide loading state of RhoA. By employing the FRET sensor we show that a plasma membrane located RhoGEF, p63RhoGEF, can rapidly activate RhoA through endogenous GPCRs and that localized RhoA activity at the cell periphery correlates with actin polymerization. Moreover, synthetic recruitment of the catalytic domain derived from p63RhoGEF to the plasma membrane, but not to the Golgi apparatus, is sufficient to activate RhoA. The synthetic system enables local activation of endogenous RhoA and effectively induces actin polymerization and changes in cellular morphology. Together, our data demonstrate that GEF activity at the plasma membrane is sufficient for actin polymerization via local RhoA signaling.
Figures


References
-
- Burridge K. & Wennerberg K. Rho and Rac take center stage. Cell 116, 167–179 (2004). - PubMed
-
- Jaffe A. B. & Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (2005). - PubMed
-
- Barrio-Real L. & Kazanietz M. G. Rho GEFs and cancer: linking gene expression and metastatic dissemination. Sci. Signal. 5, pe43 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

