DNA Topoisomerases
- PMID: 26435256
- PMCID: PMC11575854
- DOI: 10.1128/ecosalplus.ESP-0010-2014
DNA Topoisomerases
Abstract
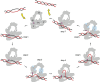
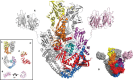
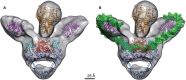
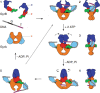
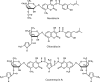
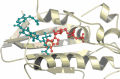
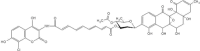
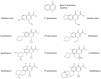
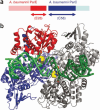
DNA topoisomerases are enzymes that control the topology of DNA in all cells. There are two types, I and II, classified according to whether they make transient single- or double-stranded breaks in DNA. Their reactions generally involve the passage of a single- or double-strand segment of DNA through this transient break, stabilized by DNA-protein covalent bonds. All topoisomerases can relax DNA, but DNA gyrase, present in all bacteria, can also introduce supercoils into DNA. Because of their essentiality in all cells and the fact that their reactions proceed via DNA breaks, topoisomerases have become important drug targets; the bacterial enzymes are key targets for antibacterial agents. This article discusses the structure and mechanism of topoisomerases and their roles in the bacterial cell. Targeting of the bacterial topoisomerases by inhibitors, including antibiotics in clinical use, is also discussed.
Figures
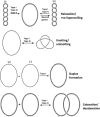
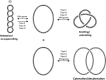
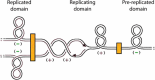
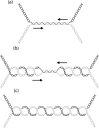


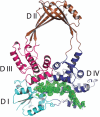
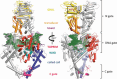
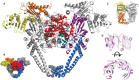

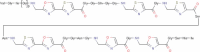
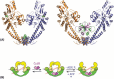

References
-
- Bates AD, Maxwell A. 2005. DNA Topology. Oxford University Press, Oxford, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

