Sphingosine-1-phosphate receptor antagonism enhances proliferation and migration of engrafted neural progenitor cells in a model of viral-induced demyelination
- PMID: 26435414
- PMCID: PMC4607760
- DOI: 10.1016/j.ajpath.2015.06.009
Sphingosine-1-phosphate receptor antagonism enhances proliferation and migration of engrafted neural progenitor cells in a model of viral-induced demyelination
Abstract
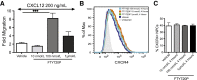
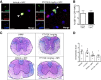
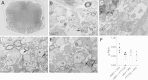
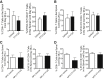
The oral drug FTY720 affects sphingosine-1-phosphate (S1P) signaling on targeted cells that bear the S1P receptors S1P1, S1P3, S1P4, and S1P5. We examined the effect of FTY720 treatment on the biology of mouse neural progenitor cells (NPCs) after transplantation in a viral model of demyelination. Intracerebral infection with the neurotropic JHM strain of mouse hepatitis virus (JHMV) resulted in an acute encephalomyelitis, followed by demyelination similar in pathology to the human demyelinating disease, multiple sclerosis. We have previously reported that intraspinal transplantation of mouse NPCs into JHMV-infected animals resulted in selective colonization of demyelinated lesions, preferential differentiation into oligodendroglia accompanied by axonal preservation, and increased remyelination. Cultured NPCs expressed transcripts for S1P receptors S1P1, S1P2, S1P3, S1P4, and S1P5. FTY720 treatment of cultured NPCs resulted in increased mitogen-activated protein kinase phosphorylation and migration after exposure to the chemokine CXCL12. Administration of FTY720 to JHMV-infected mice resulted in enhanced migration and increased proliferation of transplanted NPCs after spinal cord engraftment. FTY720 treatment did not improve clinical disease, diminish neuroinflammation or the severity of demyelination, nor increase remyelination. These findings argue that FTY720 treatment selectively increases NPC proliferation and migration but does not either improve clinical outcome or enhance remyelination after transplantation into animals in which immune-mediated demyelination is initiated by the viral infection of the central nervous system.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Glass W.G., Hickey M.J., Hardison J.L., Liu M.T., Manning J.E., Lane T.E. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol. 2004;172:4018–4025. - PubMed
-
- Marten N.W., Stohlman S.A., Bergmann C.C. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 2001;14:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

