Projections from neocortex mediate top-down control of memory retrieval
- PMID: 26436451
- PMCID: PMC4825678
- DOI: 10.1038/nature15389
Projections from neocortex mediate top-down control of memory retrieval
Abstract
Top-down prefrontal cortex inputs to the hippocampus have been hypothesized to be important in memory consolidation, retrieval, and the pathophysiology of major psychiatric diseases; however, no such direct projections have been identified and functionally described. Here we report the discovery of a monosynaptic prefrontal cortex (predominantly anterior cingulate) to hippocampus (CA3 to CA1 region) projection in mice, and find that optogenetic manipulation of this projection (here termed AC-CA) is capable of eliciting contextual memory retrieval. To explore the network mechanisms of this process, we developed and applied tools to observe cellular-resolution neural activity in the hippocampus while stimulating AC-CA projections during memory retrieval in mice behaving in virtual-reality environments. Using this approach, we found that learning drives the emergence of a sparse class of neurons in CA2/CA3 that are highly correlated with the local network and that lead synchronous population activity events; these neurons are then preferentially recruited by the AC-CA projection during memory retrieval. These findings reveal a sparsely implemented memory retrieval mechanism in the hippocampus that operates via direct top-down prefrontal input, with implications for the patterning and storage of salient memory representations.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

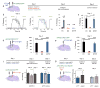




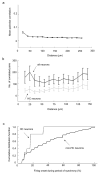
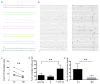
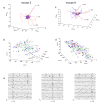





Comment in
-
Learning and memory: Influences from above on memory.Nat Rev Neurosci. 2015 Dec;16(12):703. doi: 10.1038/nrn4055. Epub 2015 Oct 28. Nat Rev Neurosci. 2015. PMID: 26507294 No abstract available.
References
-
- Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. - PubMed
-
- Han JH, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. - PubMed
-
- Han JH, et al. Selective erasure of a fear memory. Science. 2009;323:1492–1496. - PubMed
-
- Yiu AP, et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. - PubMed
-
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

