Integrin endosomal signalling suppresses anoikis
- PMID: 26436690
- PMCID: PMC4890650
- DOI: 10.1038/ncb3250
Integrin endosomal signalling suppresses anoikis
Abstract
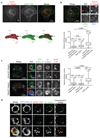
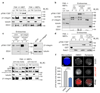
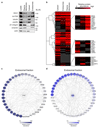
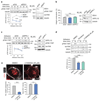
Integrin-containing focal adhesions transmit extracellular signals across the plasma membrane to modulate cell adhesion, signalling and survival. Although integrins are known to undergo continuous endo/exocytic traffic, the potential impact of endocytic traffic on integrin-induced signals is unknown. Here, we demonstrate that integrin signalling is not restricted to cell-ECM adhesions and identify an endosomal signalling platform that supports integrin signalling away from the plasma membrane. We show that active focal adhesion kinase (FAK), an established marker of integrin-ECM downstream signalling, localizes with active integrins on endosomes. Integrin endocytosis positively regulates adhesion-induced FAK activation, which is early endosome antigen-1 and small GTPase Rab21 dependent. FAK binds directly to purified endosomes and becomes activated on them, suggesting a role for endocytosis in enhancing distinct integrin downstream signalling events. Finally, endosomal integrin signalling contributes to cancer-related processes such as anoikis resistance, anchorage independence and metastasis.
Conflict of interest statement
Authors declare no conflict of interests.
Figures




Comment in
-
Endosomal integrin signals for survival.Nat Cell Biol. 2015 Nov;17(11):1373-5. doi: 10.1038/ncb3261. Nat Cell Biol. 2015. PMID: 26515017
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

