Depletion of microglia and inhibition of exosome synthesis halt tau propagation
- PMID: 26436904
- PMCID: PMC4694577
- DOI: 10.1038/nn.4132
Depletion of microglia and inhibition of exosome synthesis halt tau propagation
Abstract
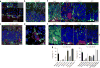
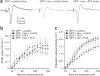
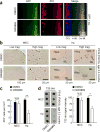
Accumulation of pathological tau protein is a major hallmark of Alzheimer's disease. Tau protein spreads from the entorhinal cortex to the hippocampal region early in the disease. Microglia, the primary phagocytes in the brain, are positively correlated with tau pathology, but their involvement in tau propagation is unknown. We developed an adeno-associated virus-based model exhibiting rapid tau propagation from the entorhinal cortex to the dentate gyrus in 4 weeks. We found that depleting microglia dramatically suppressed the propagation of tau and reduced excitability in the dentate gyrus in this mouse model. Moreover, we demonstrate that microglia spread tau via exosome secretion, and inhibiting exosome synthesis significantly reduced tau propagation in vitro and in vivo. These data suggest that microglia and exosomes contribute to the progression of tauopathy and that the exosome secretion pathway may be a therapeutic target.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Comment in
-
Microglia: Tau distributors.Nat Rev Neurosci. 2015 Dec;16(12):702. doi: 10.1038/nrn4056. Epub 2015 Oct 28. Nat Rev Neurosci. 2015. PMID: 26507296 No abstract available.
-
Alzheimer disease: Evidence for trans-synaptic and exo-synaptic tau propagation in Alzheimer disease.Nat Rev Neurol. 2015 Dec;11(12):665. doi: 10.1038/nrneurol.2015.205. Epub 2015 Nov 3. Nat Rev Neurol. 2015. PMID: 26526534 No abstract available.
References
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

