Effective intra-S checkpoint responses to UVC in primary human melanocytes and melanoma cell lines
- PMID: 26437005
- PMCID: PMC4745347
- DOI: 10.1111/pcmr.12426
Effective intra-S checkpoint responses to UVC in primary human melanocytes and melanoma cell lines
Abstract
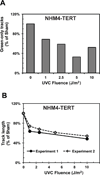
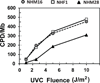
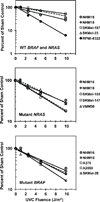
The objective of this study was to assess potential functional attenuation or inactivation of the intra-S checkpoint during melanoma development. Proliferating cultures of skin melanocytes, fibroblasts, and melanoma cell lines were exposed to increasing fluences of UVC and intra-S checkpoint responses were quantified. Melanocytes displayed stereotypic intra-S checkpoint responses to UVC qualitatively and quantitatively equivalent to those previously demonstrated in skin fibroblasts. In comparison with fibroblasts, primary melanocytes displayed reduced UVC-induced inhibition of DNA strand growth and enhanced degradation of p21Waf1 after UVC, suggestive of enhanced bypass of UVC-induced DNA photoproducts. All nine melanoma cell lines examined, including those with activating mutations in BRAF or NRAS oncogenes, also displayed proficiency in activation of the intra-S checkpoint in response to UVC irradiation. The results indicate that bypass of oncogene-induced senescence during melanoma development was not associated with inactivation of the intra-S checkpoint response to UVC-induced DNA replication stress.
Keywords: DNA replication; human; intra-S checkpoint; melanocyte; melanoma; replicon initiation; ultraviolet radiation.
© 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

