The SET Domain Is Essential for Metnase Functions in Replication Restart and the 5' End of SS-Overhang Cleavage
- PMID: 26437079
- PMCID: PMC4593633
- DOI: 10.1371/journal.pone.0139418
The SET Domain Is Essential for Metnase Functions in Replication Restart and the 5' End of SS-Overhang Cleavage
Abstract
Metnase (also known as SETMAR) is a chimeric SET-transposase protein that plays essential role(s) in non-homologous end joining (NHEJ) repair and replication fork restart. Although the SET domain possesses histone H3 lysine 36 dimethylation (H3K36me2) activity associated with an improved association of early repair components for NHEJ, its role in replication restart is less clear. Here we show that the SET domain is necessary for the recovery from DNA damage at the replication forks following hydroxyurea (HU) treatment. Cells overexpressing the SET deletion mutant caused a delay in fork restart after HU release. Our In vitro study revealed that the SET domain but not the H3K36me2 activity is required for the 5' end of ss-overhang cleavage with fork and non-fork DNA without affecting the Metnase-DNA interaction. Together, our results suggest that the Metnase SET domain has a positive role in restart of replication fork and the 5' end of ss-overhang cleavage, providing a new insight into the functional interaction of the SET and the transposase domains.
Conflict of interest statement
Figures

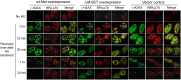
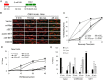


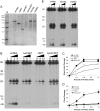

Similar articles
-
Metnase Mediates Loading of Exonuclease 1 onto Single Strand Overhang DNA for End Resection at Stalled Replication Forks.J Biol Chem. 2017 Jan 27;292(4):1414-1425. doi: 10.1074/jbc.M116.745646. Epub 2016 Dec 14. J Biol Chem. 2017. PMID: 27974460 Free PMC article.
-
The DDN catalytic motif is required for Metnase functions in non-homologous end joining (NHEJ) repair and replication restart.J Biol Chem. 2014 Apr 11;289(15):10930-10938. doi: 10.1074/jbc.M113.533216. Epub 2014 Feb 25. J Biol Chem. 2014. PMID: 24573677 Free PMC article.
-
Chk1 phosphorylation of Metnase enhances DNA repair but inhibits replication fork restart.Oncogene. 2012 Sep 20;31(38):4245-54. doi: 10.1038/onc.2011.586. Epub 2012 Jan 9. Oncogene. 2012. PMID: 22231448 Free PMC article.
-
Metnase/SETMAR: a domesticated primate transposase that enhances DNA repair, replication, and decatenation.Genetica. 2010 May;138(5):559-66. doi: 10.1007/s10709-010-9452-1. Epub 2010 Mar 23. Genetica. 2010. PMID: 20309721 Free PMC article. Review.
-
Structure, Activity, and Function of SETMAR Protein Lysine Methyltransferase.Life (Basel). 2021 Dec 4;11(12):1342. doi: 10.3390/life11121342. Life (Basel). 2021. PMID: 34947873 Free PMC article. Review.
Cited by
-
The Safe Path at the Fork: Ensuring Replication-Associated DNA Double-Strand Breaks are Repaired by Homologous Recombination.Front Genet. 2021 Sep 27;12:748033. doi: 10.3389/fgene.2021.748033. eCollection 2021. Front Genet. 2021. PMID: 34646312 Free PMC article. Review.
-
Nucleases and Co-Factors in DNA Replication Stress Responses.DNA (Basel). 2022 Mar;2(1):68-85. doi: 10.3390/dna2010006. Epub 2022 Mar 1. DNA (Basel). 2022. PMID: 36203968 Free PMC article.
-
Roles of homologous recombination in response to ionizing radiation-induced DNA damage.Int J Radiat Biol. 2023;99(6):903-914. doi: 10.1080/09553002.2021.1956001. Epub 2021 Aug 4. Int J Radiat Biol. 2023. PMID: 34283012 Free PMC article. Review.
-
SETMAR isoforms in glioblastoma: A matter of protein stability.Oncotarget. 2017 Feb 7;8(6):9835-9848. doi: 10.18632/oncotarget.14218. Oncotarget. 2017. PMID: 28038463 Free PMC article.
-
Metnase Mediates Loading of Exonuclease 1 onto Single Strand Overhang DNA for End Resection at Stalled Replication Forks.J Biol Chem. 2017 Jan 27;292(4):1414-1425. doi: 10.1074/jbc.M116.745646. Epub 2016 Dec 14. J Biol Chem. 2017. PMID: 27974460 Free PMC article.
References
-
- Fnu S, Williamson EA, De Haro LP, Brenneman M, Wray J, Shaheen M, et al. Methylation of histone H3 lysine 36 enhances DNA repair by nonhomologous end-joining. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(2):540–5. Epub 2010/12/29. 10.1073/pnas.1013571108 - DOI - PMC - PubMed
-
- Hromas R, Wray J, Lee SH, Martinez L, Farrington J, Corwin LK, et al. The human set and transposase domain protein Metnase interacts with DNA Ligase IV and enhances the efficiency and accuracy of non-homologous end-joining. DNA repair. 2008;7(12):1927–37. Epub 2008/09/09. doi: S1568-7864(08)00290-5 [pii]10.1016/j.dnarep.2008.08.002 - DOI - PMC - PubMed
-
- Kim HS, Chen Q, Kim SK, Nickoloff JA, Hromas R, Georgiadis MM, et al. The DDN catalytic motif is required for Metnase functions in non-homologous end joining (NHEJ) repair and replication restart. The Journal of biological chemistry. 2014;289(15):10930–8. 10.1074/jbc.M113.533216 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

