MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway
- PMID: 26437443
- PMCID: PMC4600732
- DOI: 10.1038/ncomms9357
MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway
Abstract
The Hippo pathway plays a central role in tissue homoeostasis, and its dysregulation contributes to tumorigenesis. Core components of the Hippo pathway include a kinase cascade of MST1/2 and LATS1/2 and the transcription co-activators YAP/TAZ. In response to stimulation, LATS1/2 phosphorylate and inhibit YAP/TAZ, the main effectors of the Hippo pathway. Accumulating evidence suggests that MST1/2 are not required for the regulation of YAP/TAZ. Here we show that deletion of LATS1/2 but not MST1/2 abolishes YAP/TAZ phosphorylation. We have identified MAP4K family members--Drosophila Happyhour homologues MAP4K1/2/3 and Misshapen homologues MAP4K4/6/7-as direct LATS1/2-activating kinases. Combined deletion of MAP4Ks and MST1/2, but neither alone, suppresses phosphorylation of LATS1/2 and YAP/TAZ in response to a wide range of signals. Our results demonstrate that MAP4Ks act in parallel to and are partially redundant with MST1/2 in the regulation of LATS1/2 and YAP/TAZ, and establish MAP4Ks as components of the expanded Hippo pathway.
Figures

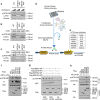

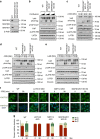
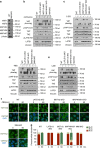
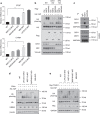


References
-
- Barry E. R. & Camargo F. D. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr. Opin. Cell Biol. 25, 247–253 (2013). - PubMed
-
- Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141, 1614–1626 (2014). - PubMed
-
- Yu F. X., Meng Z., Plouffe S. W. & Guan K. L. Hippo pathway regulation of gastrointestinal tissues. Annu. Rev. Physiol. 77, 201–227 (2014). - PubMed
-
- Piccolo S., Dupont S. & Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94, 1287–1312 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

