Respiratory syncytial virus induces phosphorylation of mTOR at ser2448 in CD8 T cells from nasal washes of infected infants
- PMID: 26437614
- PMCID: PMC4711155
- DOI: 10.1111/cei.12720
Respiratory syncytial virus induces phosphorylation of mTOR at ser2448 in CD8 T cells from nasal washes of infected infants
Abstract
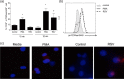
Respiratory syncytial virus (RSV)-specific CD8(+) T cell responses do not protect against reinfection. Activation of mammalian target of rapamycin (mTOR) impairs memory CD8(+) T cell differentiation. Our hypothesis was that RSV inhibits the formation of CD8(+) T cells memory responses through mTOR activation. To explore this, human and mouse T cells were used. RSV induced mTOR phosphorylation at Ser2448 in CD8 T cells. mTOR activation by RSV was completely inhibited using rapamycin. RSV-infected children presented higher mTOR gene expression on nasal washes comparing to children infected with metapneumovirus and rhinovirus. In addition, RSV-infected infants presented a higher frequency of CD8(+) pmTORser2448(+) T cells in nasal washes compared to RSV-negative infants. Rapamycin treatment increased the frequency of mouse CD8 RSV-M282-90 pentamer-positive T cells and the frequency of RSV-specific memory T cells precursors. These data demonstrate that RSV is activating mTOR directly in CD8 T cells, indicating a role for mTOR during the course of RSV infection.
Keywords: CD8+ T cells; RSV; RSV-infected infants; mTOR; nasal washes.
© 2015 British Society for Immunology.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

