PLD1 participates in BDNF-induced signalling in cortical neurons
- PMID: 26437780
- PMCID: PMC4594037
- DOI: 10.1038/srep14778
PLD1 participates in BDNF-induced signalling in cortical neurons
Abstract
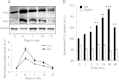
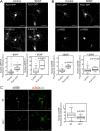
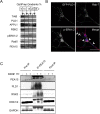
The brain-derived neurotrophic factor BDNF plays a critical role in neuronal development and the induction of L-LTP at glutamatergic synapses in several brain regions. However, the cellular and molecular mechanisms underlying these BDNF effects have not been firmly established. Using in vitro cultures of cortical neurons from knockout mice for Pld1 and Rsk2, BDNF was observed to induce a rapid RSK2-dependent activation of PLD and to stimulate BDNF ERK1/2-CREB and mTor-S6K signalling pathways, but these effects were greatly reduced in Pld1(-/-) neurons. Furthermore, phospho-CREB did not accumulate in the nucleus, whereas overexpression of PLD1 amplified the BDNF-dependent nuclear recruitment of phospho-ERK1/2 and phospho-CREB. This BDNF retrograde signalling was prevented in cells silenced for the scaffolding protein PEA15, a protein which complexes with PLD1, ERK1/2, and RSK2 after BDNF treatment. Finally PLD1, ERK1/2, and RSK2 partially colocalized on endosomal structures, suggesting that these proteins are part of the molecular module responsible for BDNF signalling in cortical neurons.
Figures



References
-
- McAllister A. K. Spatially restricted actions of BDNF. Neuron 36, 549–550 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

