Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study
- PMID: 26437782
- PMCID: PMC4705425
- DOI: 10.1038/leu.2015.270
Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study
Abstract
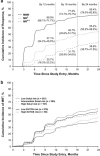
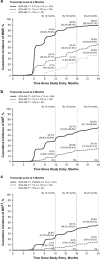
The Evaluating Nilotinib Efficacy and Safety in Clinical Trials as First-Line Treatment (ENEST1st) study included 1089 patients with newly diagnosed chronic myeloid leukemia in chronic phase. The rate of deep molecular response (MR(4) (BCR-ABL1⩽0.01% on the International Scale or undetectable BCR-ABL1 with ⩾10,000 ABL1 transcripts)) at 18 months was evaluated as the primary end point, with molecular responses monitored by the European Treatment and Outcome Study network of standardized laboratories. This analysis was conducted after all patients had completed 24 months of study treatment (80.9% of patients) or discontinued early. In patients with typical BCR-ABL1 transcripts and ⩽3 months of prior imatinib therapy, 38.4% (404/1052) achieved MR(4) at 18 months. Six patients (0.6%) developed accelerated or blastic phase, and 13 (1.2%) died. The safety profile of nilotinib was consistent with that of previous studies, although the frequencies of some nilotinib-associated adverse events were lower (for example, rash, 21.4%). Ischemic cardiovascular events occurred in 6.0% of patients. Routine monitoring of lipid and glucose levels was not mandated in the protocol. These results support the use of frontline nilotinib, particularly when achievement of a deep molecular response (a prerequisite for attempting treatment-free remission in clinical trials) is a treatment goal.
Conflict of interest statement
Authors declare the following relationships with pharmaceutical companies: Novartis—receipt of honoraria (AHo, GR, NC, JS, PlC, TM, LG, WWJ, DR, DC, TB, GS, MM, FXM, MB, FG), research funding (all authors), nonfinancial support (GR, WWJ, DC, TB), employment (PS, PDM, AP, LD) and stock ownership (PS); Pfizer—receipt of honoraria (AHo, GR, JS, PlC, DR, DC, TB, GS, FXM, MB), research funding (AHo, GR, JS, TB) and nonfinancial support (GR, TB); Ariad—receipt of honoraria (AHo, GR, NC, JS, PlC, DR, TB, GS, MM, FXM, MB), research funding (AHo, GR, JS, MM) and nonfinancial support (GR, TB); Bristol-Myers Squibb—receipt of honoraria (AHo, GR, JS, PlC, TM, DR, DC, TB, GS, MM, FXM, MB), research funding (AHo, GR, JS, MM) and nonfinancial support (GR, TB).
Figures

References
-
- 1Tasigna [package insert]. Novartis Pharmaceuticals Corporation: East Hanover, NJ, USA, 2015.
-
- 3Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia 2012; 26: 2197–2203. - PubMed
-
- 4Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol 2011; 12: 841–851. - PubMed
-
- 5Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

