Copper Transport Protein Antioxidant-1 Promotes Inflammatory Neovascularization via Chaperone and Transcription Factor Function
- PMID: 26437801
- PMCID: PMC4594038
- DOI: 10.1038/srep14780
Copper Transport Protein Antioxidant-1 Promotes Inflammatory Neovascularization via Chaperone and Transcription Factor Function
Abstract
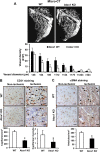
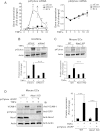
Copper (Cu), an essential micronutrient, plays a fundamental role in inflammation and angiogenesis; however, its precise mechanism remains undefined. Here we uncover a novel role of Cu transport protein Antioxidant-1 (Atox1), which is originally appreciated as a Cu chaperone and recently discovered as a Cu-dependent transcription factor, in inflammatory neovascularization. Atox1 expression is upregulated in patients and mice with critical limb ischemia. Atox1-deficient mice show impaired limb perfusion recovery with reduced arteriogenesis, angiogenesis, and recruitment of inflammatory cells. In vivo intravital microscopy, bone marrow reconstitution, and Atox1 gene transfer in Atox1(-/-) mice show that Atox1 in endothelial cells (ECs) is essential for neovascularization and recruitment of inflammatory cells which release VEGF and TNFα. Mechanistically, Atox1-depleted ECs demonstrate that Cu chaperone function of Atox1 mediated through Cu transporter ATP7A is required for VEGF-induced angiogenesis via activation of Cu enzyme lysyl oxidase. Moreover, Atox1 functions as a Cu-dependent transcription factor for NADPH oxidase organizer p47phox, thereby increasing ROS-NFκB-VCAM-1/ICAM-1 expression and monocyte adhesion in ECs inflamed with TNFα in an ATP7A-independent manner. These findings demonstrate a novel linkage between Atox1 and NADPH oxidase involved in inflammatory neovascularization and suggest Atox1 as a potential therapeutic target for treatment of ischemic disease.
Figures






References
-
- Brewer G. J. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discov Today 10, 1103–1109, (2005) - PubMed
-
- Harris E. D. A requirement for copper in angiogenesis. Nutr Rev 62, 60–64 (2004). - PubMed
-
- Goodman V. L., Brewer G. J. & Merajver S. D. Copper deficiency as an anti-cancer strategy. Endocr Relat Cancer 11, 255–263 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

