Intraspinal serotonergic neurons consist of two, temporally distinct populations in developing zebrafish
- PMID: 26437856
- PMCID: PMC4823180
- DOI: 10.1002/dneu.22352
Intraspinal serotonergic neurons consist of two, temporally distinct populations in developing zebrafish
Abstract
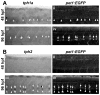
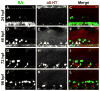
Zebrafish intraspinal serotonergic neuron (ISN) morphology and distribution have been examined in detail at different ages; however, some aspects of the development of these cells remain unclear. Although antibodies to serotonin (5-HT) have detected ISNs in the ventral spinal cord of embryos, larvae, and adults, the only tryptophan hydroxylase (tph) transcript that has been described in the spinal cord is tph1a. Paradoxically, spinal tph1a is only expressed transiently in embryos, which brings the source of 5-HT in the ISNs of larvae and adults into question. Because the pet1 and tph2 promoters drive transgene expression in the spinal cord, we hypothesized that tph2 is expressed in spinal cords of zebrafish larvae. We confirmed this hypothesis through in situ hybridization. Next, we used 5-HT antibody labeling and transgenic markers of tph2-expressing neurons to identify a transient population of ISNs in embryos that was distinct from ISNs that appeared later in development. The existence of separate ISN populations may not have been recognized previously due to their shared location in the ventral spinal cord. Finally, we used transgenic markers and immunohistochemical labeling to identify the transient ISN population as GABAergic Kolmer-Agduhr double-prime (KA″) neurons. Altogether, this study revealed a novel developmental paradigm in which KA″ neurons are transiently serotonergic before the appearance of a stable population of tph2-expressing ISNs.
Keywords: Kolmer-Agduhr; pet1; serotonin; spinal cord; tryptophan hydroxylase.
© 2015 Wiley Periodicals, Inc.
Figures






References
-
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–664. - PubMed
-
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG, Monaco PJ. Adverse effects of serotonin depletion in developing zebrafish. Neurotoxicol Teratol. 2012;34:152–160. - PubMed
-
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. - PubMed
-
- Bellipanni G, Rink E, Bally-Cuif L. Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Mech Dev. 2002;119(Suppl 1):S215–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

