Effects of prenatal inhalation exposure to copper nanoparticles on murine dams and offspring
- PMID: 26437892
- PMCID: PMC4594905
- DOI: 10.1186/s12989-015-0105-5
Effects of prenatal inhalation exposure to copper nanoparticles on murine dams and offspring
Abstract
Background: Increasing numbers of individuals may be exposed to nanomaterials during pregnancy. The overarching goal of this investigation was to determine if prenatal inhalation exposure to copper nanoparticles (Cu NPs) has an effect on dams and offspring, including an analysis of inflammatory markers (Th1/Th2 cytokine profiles).
Methods: Physicochemical characterization of Cu NPs was performed. Pregnant and non-pregnant mice (C57Bl/6 J) were exposed to Cu NPs or laboratory air in the whole-body chamber for 4 hrs/day on gestation days (GD) 3-19 (3.5 mg/m(3)). Animals were euthanized on GD 19 (0 week) or 7 weeks later. Bronchoalveolar lavage (BAL) fluid was analyzed for total and differential cells. Cytokine/chemokine concentrations were determined in the BAL fluid and the plasma of dams/non-pregnant mice and pups. Cu content was determined in the lungs and the blood of dams/non-pregnant mice and pups, in the placentas as well as in the whole bodies of pups immediately after delivery. Lungs and placentas were evaluated for histopathological changes. Gene expression of the Th1/Th2 profiles were analyzed in spleens of pups.
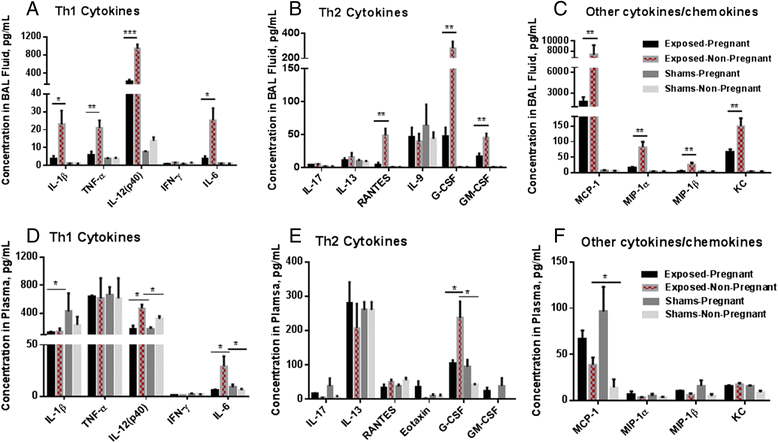
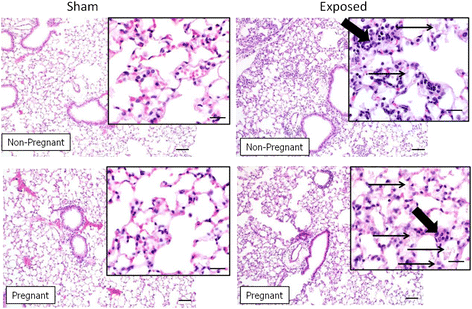
Results: The survival rate of 7 week old pups exposed to Cu NPs was significantly lower than control pups (73 vs. 97 %). The average litter size, male/female ratio, body weight and lenght at birth were not different between Cu NP-exposed and control mice. Both pregnant and non-pregnant mice exposed to Cu NPs had significant pulmonary inflammation with increased number of neutrophils in the BAL fluid compared to controls. Perivascular lymphoplasmacytic cuffing was found in the lungs of exposed mice and was more pronounced in the non-pregnant group. Similarly, levels of inflammatory cytokines/chemokines IL-12(p40), G-CSF, GM-CSF, KC, MCP-1, MIP-1α, MIP-1β, RANTES and TNF-α in BAL fluid were significantly higher in non-pregnant than pregnant exposed mice. Histopathology evaluation of placentas did not identify any pathological changes. No translocation of Cu into the placenta or the fetus was found by inductively coupled plasma-mass spectroscopy. Expression of several Th1/Th2 or other immune response genes in pups' spleens were found to be significantly up- or down-regulated.
Conclusions: Prenatal exposure to Cu NPs caused a profound pulmonary inflammation in dams and strong immunomodulatory effects in offspring. There was no clear polarization of genes expressed in pups' spleens towards Th1 or Th2 type of response.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

