Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial
- PMID: 26438038
- PMCID: PMC4595062
- DOI: 10.1186/s12931-015-0281-8
Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial
Abstract
Iota-carrageenan (I-C) is active against respiratory viruses in vitro and was effective as nasal spray in three previous clinical trials. The current trial served to further investigate I-C in patients with early common cold symptoms.
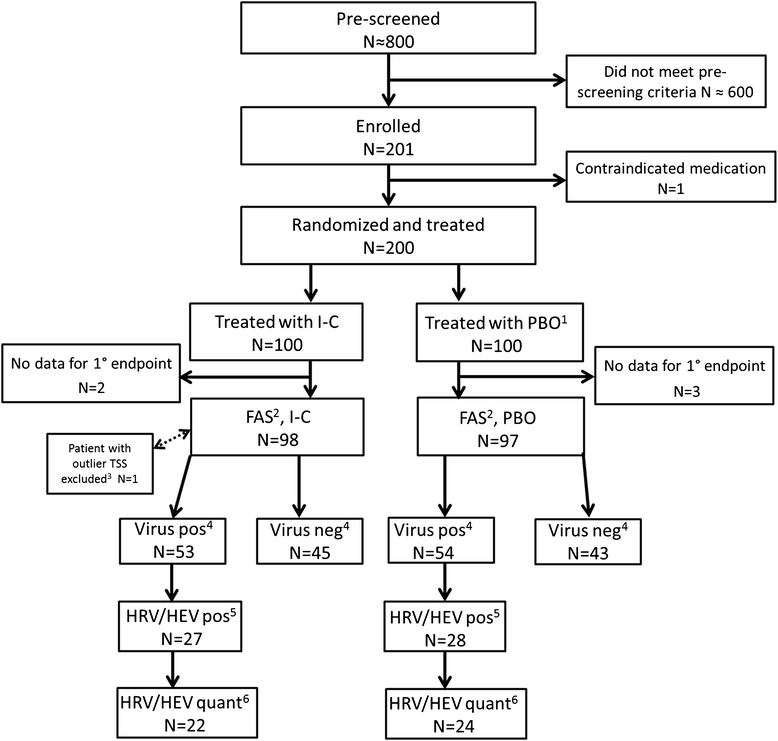
Methods: This randomized, placebo-controlled, double-blind phase IV trial was conducted in 200 adult patients with self-diagnosed colds of <48 h' duration that were confirmed by baseline cold symptom scores. Patients were to self-administer 0.12 % I-C or placebo spray (NaCl 0.5 %) four times daily for four to ten days and record symptom information for ten days. Common respiratory viruses were quantified by RT-PCR during pretreatment and on Day 3 or 4. The primary endpoint was the mean total symptom score (TSS) of eight cold symptoms on Days 2-4 (TSS2-4).
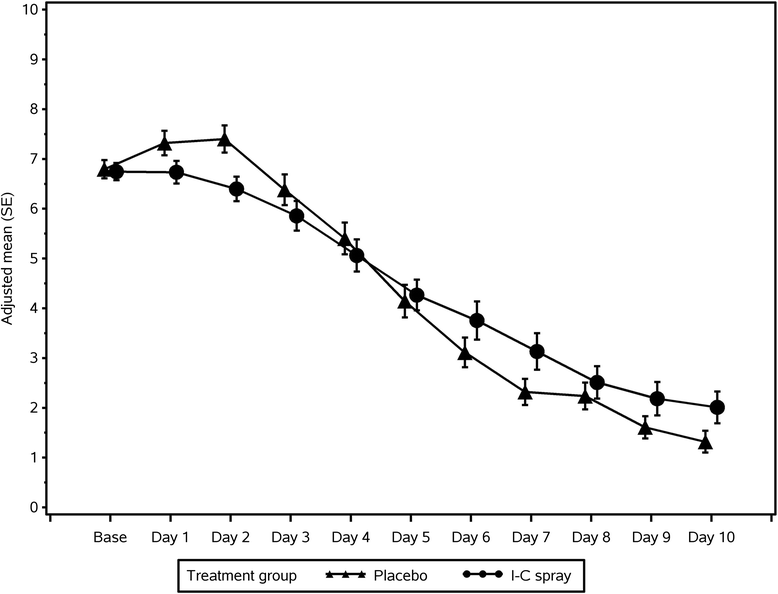
Results: Patients in both treatment groups had similar baseline TSSs (mean TSS: 6.75 for I-C and 6.79 for placebo). Viruses were detected in baseline samples from 53 of 98 I-C patients (54.1 %) and 54 of 97 placebo patients (55.7 %). Mean ± SE for TSS2-4 was 5.78 ± 0.25 for I-C patients and 6.39 ± 0.25 for placebo (p = 0.0895). Exploratory analyses after unblinding (TSS2-4 excluding a patient with aberrantly high symptom scores [TSS2-4, ex 1pt]; mean of TSS over Days 1-4 [TSS1-4]; change in TSS1-4 relative to baseline [TSS1-4, rel]) demonstrated treatment differences in favor of I-C (p = 0.0364, p = 0.0495 and p = 0.0421, respectively). For patients with quantifiable rhinovirus/enterovirus at baseline, there was a trend towards greater reduction of virus load at Day 3 or 4 (p = 0.0958; I-C: 90.2 % reduction in viral load; placebo: 72.0 %). Treatments were well tolerated with no differences in adverse event rates.
Conclusions: The primary endpoint did not demonstrate a statistically significant difference between I-C and placebo but showed a trend towards I-C benefit. Exploratory analyses indicated significant reduction of cold symptoms in the I-C group relative to placebo during the first four days when symptoms were most severe, and also substantiated I-C's activity against rhinovirus/enterovirus.
Trial registration: NCT01944631 (clinicaltrials.gov).
Figures
References
-
- Fendrick MA, Monto AS, Nightengale B, Sarnes M. The Economic Burden of Non–Influenza-Related Viral Respiratory Tract Infection in the United States. Arch Intern Med. 2003;163:487–94. http://dx.doi.org/10.1001/archinte.163.4.487. - DOI - PubMed
-
- Leibbrandt A, König-Schuster M, Weinmüllner R, Kalthoff D, Pflugfelder B, Graf P, et al. Iota-Carrageenan is a potent inhibitor of influenza A virus infection. PLoS One. 2010;5:e14320. http://dx.doi.org/10.1371/journal.pone.0014320. - DOI - PMC - PubMed
-
- Grassauer A, Weinmuellner R, Meier C, Pretsch A, Prieschl-Grassauer E, Unger H. Iota-carrageenan is a potent inhibitor of rhinovirus infection. Virol J. 2008;5:107. http://dx.doi.org/10.1186/1743-422×-5-107. - DOI - PMC - PubMed
-
- Eccles R, Meier C, Jawad M, Weinmueller R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res (Lond). 2010;11:108. http://dx.doi.org/10.1186/1465-9921-11-108 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

